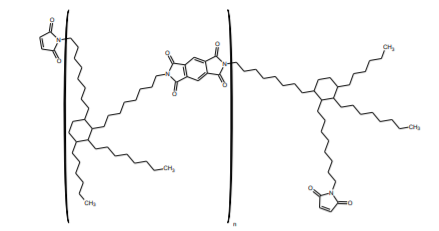
BMI-6000 Imide-extended Bismaleimide Oligomer
- Superior thermal stability
- Good dielectric properties
- Toughener
Product Description
BMI-6000 has been designed as a curable, low DK and Df alternative to replace both the Kapton and adhesive layers in the manufacture of FCCL materials. The material has excellent thermal stability and workability. It is soluble in a variety of solvents such as cyclopentanone, cyclohexanone, MEK, DMF, DMAC, and NMP in combination with aromatic solvents. It can be processed in a resin system as a solid or dissolved in a solvent.
BMI-6000 is recommended for use as an adhesive layer when laminating materials for FCCL applications. Adhesion promoters, solvent(s), are required for successful use. BMI6000 has much higher Tg than products such as BMI-689 and require thermal curing to obtain the published values. This material is not suitable for UV curing.
Aromatic BMI resins lose some of their user-friendly nature compared to the aliphatic versions: they are less soluble in normal solvents, less compatible with other resins, require more complicated curing processes, etc.
Technical Specifications
| General Properties | |||||
| Appearance Appearance Appearance at room temperature. | Light yellow | ||||
| Thermal Properties | |||||
| |||||
| Glass Transition Temperature (Tg) Glass Transition Temperature (Tg) The glass transition temperature for organic adhesives is a temperature region where the polymers change from glassy and brittle to soft and rubbery. Increasing the temperature further continues the softening process as the viscosity drops too. Temperatures between the glass transition temperature and below the decomposition point of the adhesive are the best region for bonding. The glass-transition temperature Tg of a material characterizes the range of temperatures over which this glass transition occurs. | 214 °C | ||||
Additional Information
What are the main differences with BMI 6100?
- The reaction to form the product is performed in anisole and for economy, we prefer to leave it in anisole
- We are unable to precipitate this higher molecular weight material to form a solid powder - it forms a gooey mess.