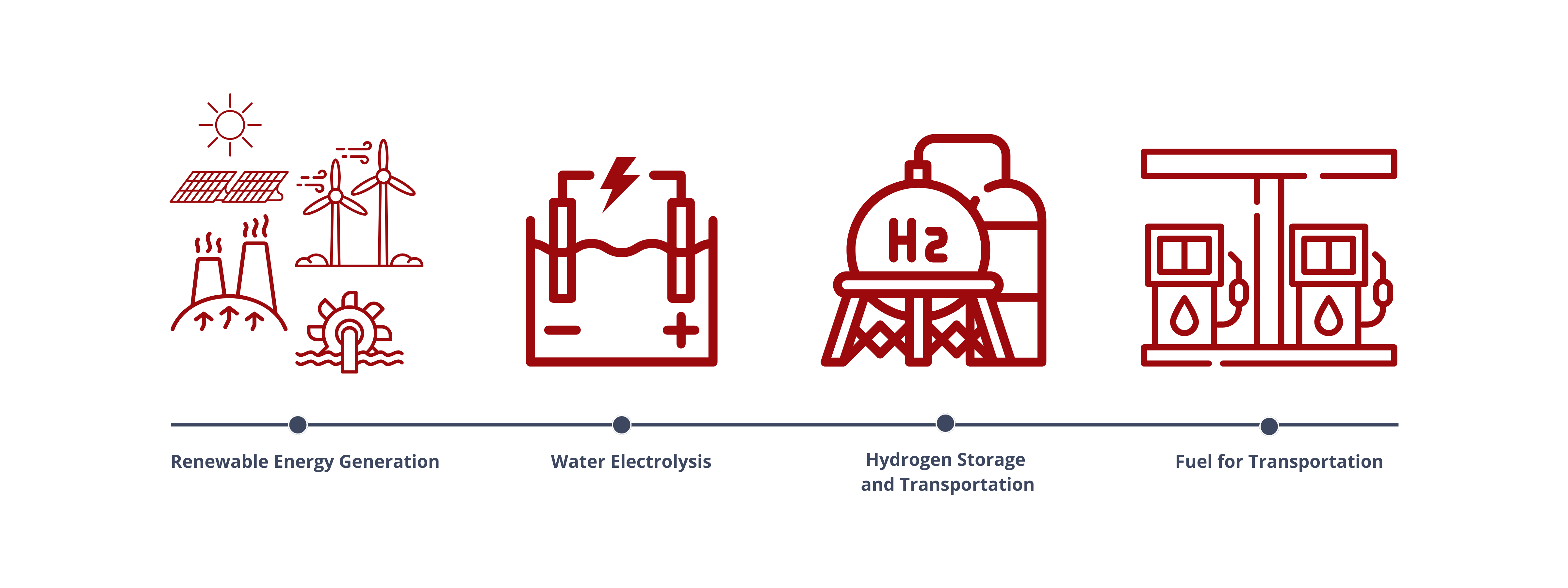
Water Electrolyzers
Frequently Asked Questions about Water Electrolyzers
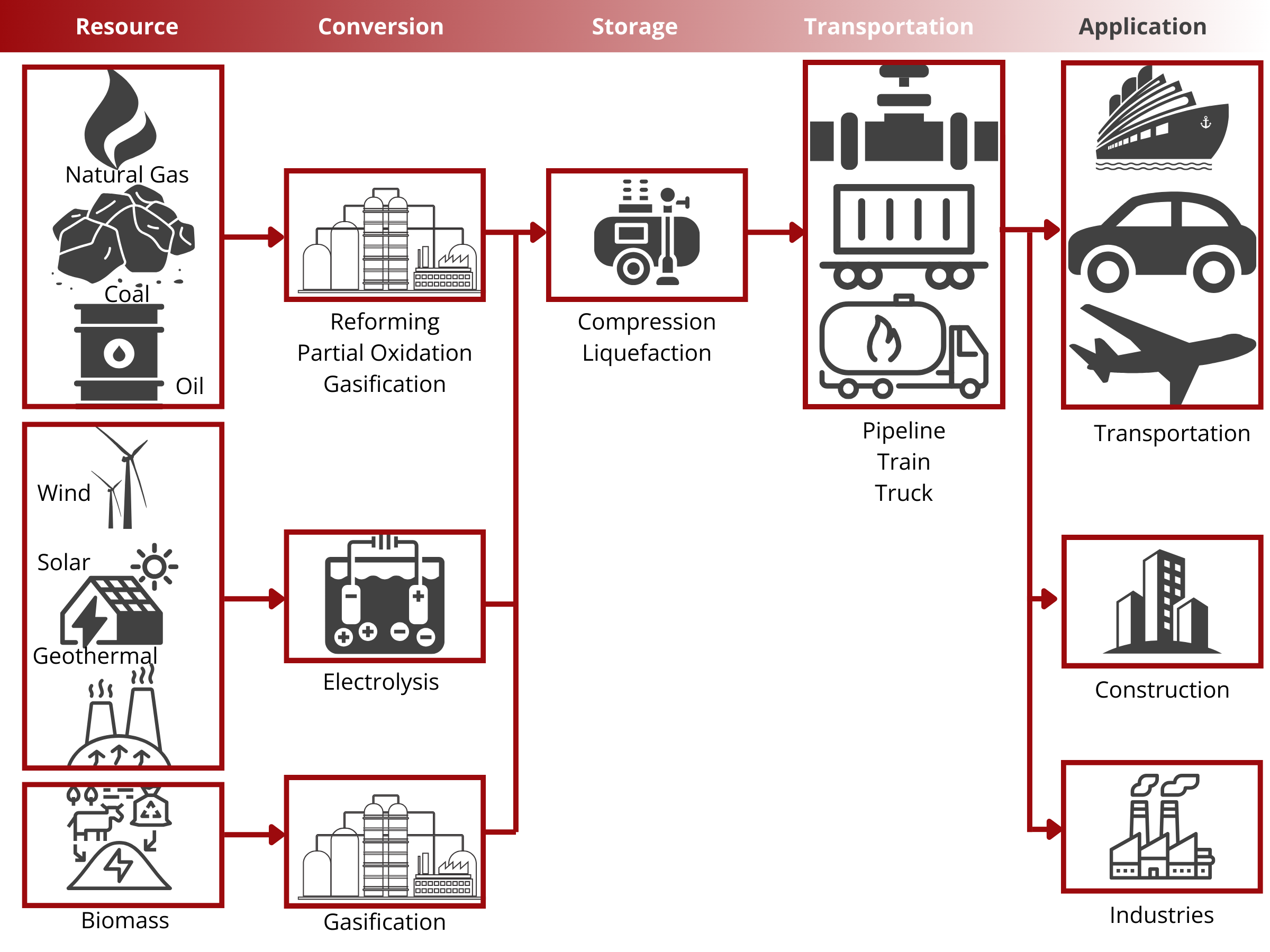
Gas Diffusion Layers (GDLs) are crucial components in water electrolyzers, serving as porous substrates that facilitate efficient gas transport and distribution at the electrode interface. Carbon sheets are typically utilized on the cathode side for their electrochemical stability, high porosity, and hydrophobic nature. These properties ensure uniform gas distribution and prevent flooding of the cathode electrode during electrolysis.
On the anode side, metal-based Porous Transport Layers (PTLs) such as nickel, stainless steel, and titanium are commonly used. These materials offer corrosion resistance, mechanical strength, and high electrical conductivity, making them suitable for the oxidative environment of the anode compartment. Metal PTLs provide structural support to the anode electrode, enable efficient electron transfer, and ensure long-term durability under harsh operating conditions such as high temperatures and corrosive electrolytes.
How does porosity and pore size affect the performance of Porous Transport layer (PTLs) in water electrolyzers?
the porosity has a direct effect on the contact resistance between the MEA and the PTL. A small porosity increases the contact surface but hinders the oxygen expelling. On the other hand, for a given porosity, large pores facilitate gas/water transport but will increase the electrical resistance since contact points (forming the contact surface) are more distant from each other. Conversely, small pores will improve the electrical contact but gas expelling and water feeding will be obstructed, increasing the mass transport losses.
Untreated metal porous felts will not be consumed like a carbon Gas Diffusion Layer (GDL) will. However, the presence of oxygen does have an impact if the electrolyzer is being operated at high pressures (1 bar to 3 bars). The untreated metal surface will quickly form an electrically insulated oxide layer on the surface of the small-diameter fibers under high O2 pressures. This oxide coating will eventually affect the efficiency of the overall system acting as an electrical insulator and it will increase the interfacial resistance in the cell, lowering the electrochemical performance.
To prevent the formation of this detrimental oxide coating, applying a gold or platinum coating is recommended. This coating extends the lifetime of the metal felts, making it well-suited for applications requiring both high performance and long-term reliability. By preventing the formation of an oxide coating, stability in the electrochemical performance of the electrolyzer is greatly enhanced, ensuring consistent and efficient operation over its lifespan.
What is the difference between 1600°C and 2000°C graphitization?
Graphitization at temperatures around 2000°C typically results in enhanced electrical properties in carbon papers. However, this improvement often accompanies trade-offs, such as reduced compressibility or increased brittleness. Finding the optimal balance depends on understanding the specific requirements of your application.
Presentations
Cost Analysis of Water Electrolyzers
The presentation shown on the left is focused on explaining the major costs involved in the production of water electrolyzers, which are critical for hydrogen production through water electrolysis. It highlights the various expense categories that contribute to the final production cost of hydrogen, including capital expenditures on equipment and infrastructure, operational costs, and the significant impact of energy consumption. Capital costs are heavily influenced by the materials used in constructing the electrolyzer components, such as electrodes and membranes, which often include expensive metals like platinum and iridium. Opportunities for reducing these costs lie in material science innovations that could offer cheaper alternatives or methods to decrease material usage without sacrificing performance.
On the operational side, the presentation outlines maintenance and labor costs, as well as the pivotal role of energy expenses, which constitute the majority of ongoing operational outlays due to the intensive energy requirements of the electrolysis process. The efficiency with which an electrolyzer operates directly impacts the amount of electricity needed, with more efficient systems reducing overall energy costs. Additionally, as renewable energy sources become more accessible and cost-effective, their integration into hydrogen production could further drive down energy expenses. This analysis not only aids in pinpointing potential areas for technological advancements and cost reductions but also underscores the critical role of market conditions and policy frameworks in advancing hydrogen as a sustainable alternative fuel source.
Fundamental Principles of Water Electrolyzers and Fuel Cells
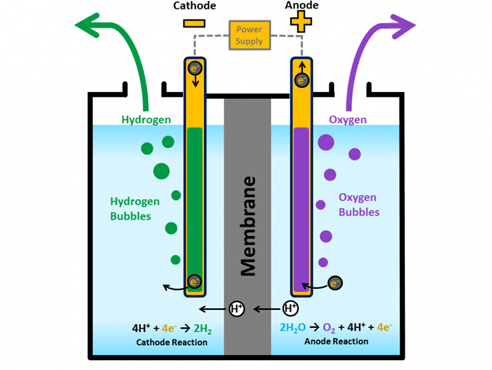
The presentation shown on the left delves into the fundamental principles of electrolyzers and fuel cells, offering a comprehensive understanding of how these technologies function to produce and utilize hydrogen energy. For electrolyzers, the focus is on the electrochemical process that splits water into hydrogen and oxygen using electricity. This section explains the key components such as the cathode, anode, and electrolyte, and how they interact to facilitate the movement of ions and electrons. The discussion extends to the factors affecting electrolyzer efficiency, including temperature, pressure, and catalyst material, which are crucial for optimizing the system's performance and overall energy efficiency.
In the section on fuel cells, the presentation explores how these devices reverse the process conducted by electrolyzers by converting hydrogen back into electricity through a reaction with oxygen. It outlines the basic structure of a fuel cell, including the anode, cathode, and electrolyte, and describes the flow of ions and electrons across the cell. The presentation further elaborates on the different types of fuel cells, such as PEM (Proton Exchange Membrane) and SOFC (Solid Oxide Fuel Cell), highlighting their unique applications and efficiency profiles. This portion also addresses challenges like durability and cost, providing insights into ongoing research aimed at making fuel cell technology more practical and affordable for widespread use. The thorough explanation of these principles underscores their potential in contributing to a cleaner energy landscape and the importance of continued innovation and investment in these technologies.
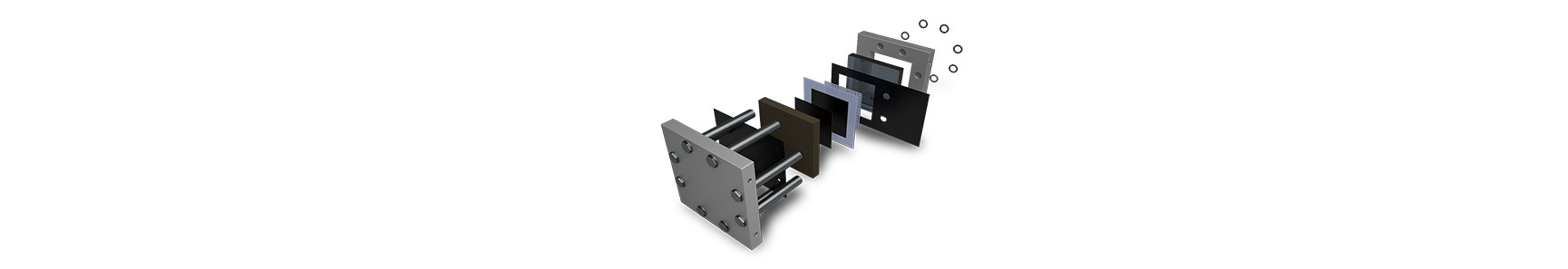
Overview of Materials used in an electrolyzer
The presentation shown on the left offers an in-depth look at the materials used in the manufacture of electrolyzers, a key component in hydrogen production through water electrolysis. It explores various materials essential for constructing the core parts of an electrolyzer, such as electrodes, electrolyte, and membrane. The choice of these materials critically affects the efficiency, durability, and cost of the electrolyzers. For electrodes, materials such as platinum and iridium are commonly used due to their excellent catalytic properties; however, their high cost and scarcity prompt ongoing research into less expensive alternatives like nickel or iron-based catalysts.
The presentation also highlights the importance of the membrane material, which needs to conduct ions effectively while preventing the mixing of hydrogen and oxygen gases produced during electrolysis. Materials like Nafion, a sulfonated tetrafluoroethylene-based fluoropolymer-copolymer, are typically used for their strong ionic conductivity and chemical stability. Advances in membrane technology that reduce costs or enhance performance could significantly impact the overall efficiency and affordability of hydrogen production. Additionally, the use of advanced ceramics and composites in the structural components of electrolyzers is discussed, emphasizing their role in withstanding the harsh operational environments of high temperatures and corrosive conditions. This comprehensive review of materials not only underlines the challenges faced in material selection but also points towards potential areas for innovation in the quest to optimize and economize green hydrogen production.