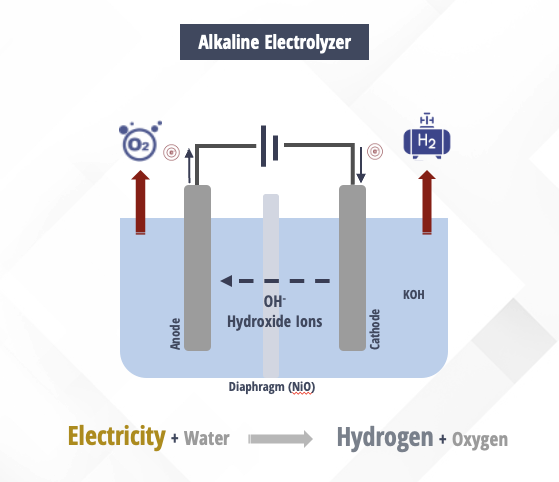
Alkaline Electrolyzers
Electrolysis is the process of using electricity to split water into hydrogen and oxygen. This process is key to the production of hydrogen, which is used in fuel cells for power generation, transportation, and other applications. There are several types of electrolyzers available on the market, including alkaline electrolyzers. In this application page, we will explore the advantages of alkaline electrolyzers compared to other types of electrolyzers, the main differences between them, and the materials used in their construction. Please note that these are general principles and not set in stone.
Alkaline Electrolyzers
Uses a liquid electrolyte solution such as potassium hydroxide (KOH) or sodium hydroxide (NAOH), and water.
The hydrogen is produced in a “cell” which consists of an anode, cathode and membrane. The cells are typically assembled in series in a “cell stack” that produces more hydrogen and oxygen as the amount of cell increases.
When current is applied on the cell stack, the hydroxide ions (OH-) move through the electrolyte from the cathode to the anode of each cell, with hydrogen gas bubbles generated on the cathode side of the electrolyzer and oxygen gas at the anode
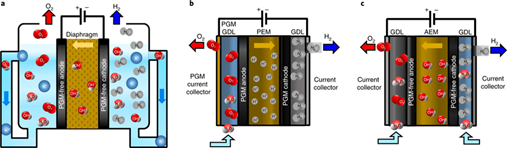
Operating principles of Alkaline Electrolyzers
An alkaline electrolyzer is a device that uses an electrolyte solution, typically potassium or sodium hydroxide, to split water molecules into hydrogen and oxygen through a process called electrolysis. The chemical principle behind an alkaline electrolyzer is based on the principles of electrochemistry. In electrochemistry, chemical reactions are driven by the transfer of electrons from one substance to another. When an electrical current is applied to an electrolyte solution, it causes the electrolyte solution to undergo a process called electrolysis. During this process, the electrical current causes the water molecules to be split into their constituent atoms of hydrogen and oxygen.
In an alkaline electrolyzer, the process is made possible by the presence of the electrolyte solution, which contains hydroxide ions (OH-) that facilitate the transfer of electrons between the electrodes and the water molecules. The hydroxide ions are attracted to the positively charged anode (the electrode connected to the positive terminal of the power source) and the hydrogen ions (H+) are attracted to the negatively charged cathode (the electrode connected to the negative terminal of the power source).
- At the anode, water molecules are oxidized to form oxygen gas and positively charged hydrogen ions: 2H2O → O2 + 4H+ + 4e-
- At the cathode, hydrogen ions are reduced to form hydrogen gas: 4H+ + 4e- → 2H2
- Overall, the reaction can be expressed as: 2H2O → 2H2 + O2
The electrolyte solution plays an important role in the process by providing a conductive medium for the flow of electrons between the electrodes and the water molecules. The hydroxide ions in the electrolyte solution also help to maintain a stable pH level, which is important for the efficient functioning of the electrolyzer.
In summary, an alkaline electrolyzer works by using an electrolyte solution to facilitate the transfer of electrons between the electrodes and the water molecules, resulting in the production of hydrogen and oxygen gas.
Main Differences Between Alkaline Electrolyzers and Other Types of Electrolyzers
There are several key differences between alkaline electrolyzers and other types of electrolyzers, including:
Electrolyte: Alkaline electrolyzers use a liquid potassium hydroxide (KOH) electrolyte, whereas other types of electrolyzers use solid polymer electrolytes or acidic electrolytes.
Operating temperature: Alkaline electrolyzers operate at higher temperatures than other types of electrolyzers, typically between 70°C and 100°C.
Efficiency: Alkaline electrolyzers have a higher energy efficiency than other types of electrolyzers, meaning they require less energy to produce a given amount of hydrogen.
Hydrogen purity: Alkaline electrolyzers can produce high-purity hydrogen without the need for additional purification steps.
Materials Used in Alkaline Electrolyzers
Alkaline electrolyzers are typically constructed using materials that are resistant to corrosion and can withstand the harsh conditions of the electrolysis process. The main materials used in alkaline electrolyzers include:
Electrodes: The electrodes in alkaline electrolyzers are typically made of nickel, nickel-plated steel, or titanium coated with a nickel-based alloy. These materials are resistant to corrosion and can withstand the harsh conditions of the electrolysis process.
Membrane: Alkaline electrolyzers use a liquid KOH electrolyte, so they do not require a membrane.
Cell components: The cell components in alkaline electrolyzers, such as cell frames, separators, and pressure vessels, are typically made of materials such as stainless steel, carbon steel, or nickel alloys. These materials are resistant to corrosion and can withstand the harsh conditions of the electrolysis process.
Alkaline electrolyzers are a cost-effective and efficient option for hydrogen production. They have several advantages over other types of electrolyzers, including higher energy efficiency, low cost, high purity hydrogen production, and mechanical robustness. Alkaline electrolyzers are constructed using materials that are resistant to corrosion and can withstand the harsh conditions of the electrolysis process, such as nickel, nickel-plated steel, titanium coated with a nickel-based alloy, stainless steel, carbon steel, and nickel alloys. By utilizing alkaline electrolyzers, industries can produce high-purity hydrogen more efficiently and at a lower cost, contributing to a more sustainable and clean energy future.
Can you use Carbon papers in Alkaline Electrolyzers?
Carbon papers can be used as electrode materials in alkaline electrolyzers, but they are not typically used as Gas Diffusion Layers (GDLs) in alkaline electrolysis.
In alkaline electrolysis, the electrolyte is a concentrated solution of a strong base, such as potassium hydroxide (KOH) or sodium hydroxide (NaOH), and the reaction occurs at a higher pH compared to PEM electrolysis. The high pH environment can cause the carbon fibers in the carbon paper to degrade over time, which can negatively impact the performance and durability of the electrolyzer.
Instead, in alkaline electrolysis, metal mesh screens or porous ceramic materials are often used as the electrode support and GDL material. These materials are more resistant to the high pH environment and can provide similar porosity and conductivity to carbon paper.
It is important to select the appropriate electrode and GDL material based on the specific operating conditions and performance requirements of the alkaline electrolyzer. Factors such as the concentration of the electrolyte, temperature, and pressure can also impact the selection of materials for the alkaline electrolyzer.

