Fuel Cells
Fuel cells. No matter how familiar you are with them, reading their name always brings images from a distant future. They’ve been used in numerous sci-fi movies, books and video games to portray clean and limitless energy that powers up the cities of the future and our martian colonies. They’ve also been a highly controversial news topic since they have been the technology of the future for the last 30 years. As controversial as this sounds, it is absolutely true. They’ve been around since 1839 with various iterations coming in and out of fashion.
Fuel cells are being used in pilot projects and can power up high performance cars and applications. But why are they still “under development”? What keeps them from being the technology of the future and what can we do to bring the future closer to us? That’s what we are going to discuss in this quick ELI5 of fuel cells. What are they? How do they work? What kinds of products and technologies are coming into play to create energy out of thin air?
Like batteries, fuel cells are energy converters – they use an electrochemical reaction to take the chemical energy stored in a fuel source and convert it to electricity. Unlike batteries, which contain a fixed supply of energy, fuel cells do not require recharging. As long as fuel is continuously supplied to the fuel cell, electricity, water and heat will be produced
How does a fuel cell work?
So how does a Fuel cell work? In principle the concept of a fuel cell is pretty simple.
- Hydrogen gets in from one side
- Oxygen comes in from the other side
- A couple of reduction and oxidation reactions happen within the system
- Electricity and other byproducts such as heat and water come out.
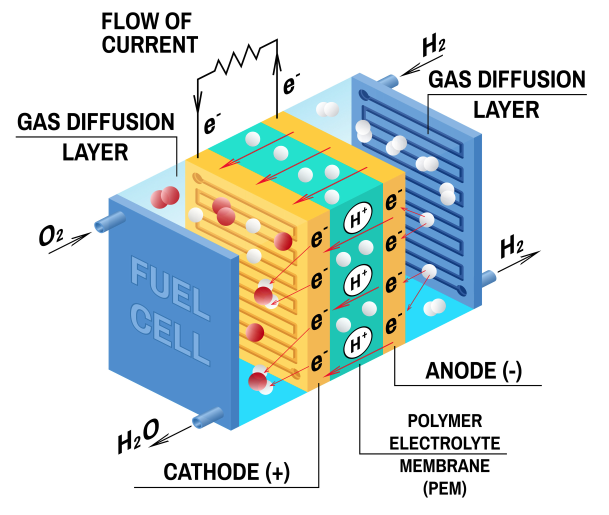
To expand a little bit on #3, atoms of hydrogen are stripped from their electrons at the Anode side while positive anions or protons (depending on the membrane) pass from the cathode side. The negative electrons are squeezed through the circuit and generate electricity.
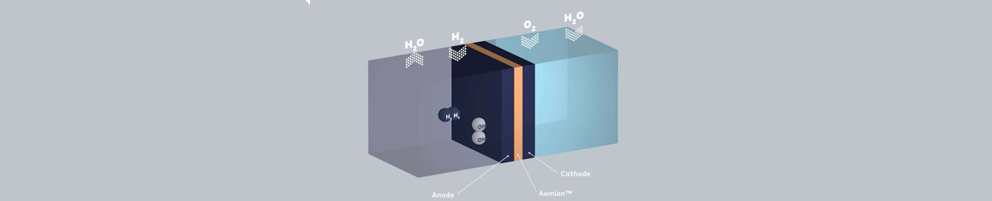
A fuel cell is comprised of two electrodes and an electrolyte membrane. The electrodes are called a cathode and an anode, and they sandwich the electrolyte membrane between them. Within that system, a series of chemical reactions occur to separate the electrons from the fuel molecules to create energy.
The fuel, typically hydrogen, is fed into the anode on one side while oxygen is fed into the cathode on the other. At the anode, the hydrogen fuel molecules are separated into protons and electrons that will travel different paths toward the cathode. The electrons go through the electrical circuit, creating the flow of electricity. The protons travel through the electrolyte to the cathode. Once at the cathode, oxygen molecules react with the electrons and with the protons to create water molecules.
A fuel cell is a clean energy source with the only byproducts being electricity (power), heat and water. A single fuel cell alone only produces a few watts of power; therefore, several fuel cells can be stacked together to create a fuel cell stack. When combined in stacks, the fuel cells’ output can vary greatly, from just a few kilowatts of power to multi-megawatt installations.
You can generalize and say that it is a kind of battery that makes electricity on the fly. This is an example for the sake of simplicity. You can replace Hydrogen with any other suitable fuel and Oxygen with any other suitable oxidizing agent and you’d get the same result. Also what comes in/out depends on the type of Ion exchange membrane and catalyst that we are going to use.
There are various fuel cell stacks consisting of 3, 5 or even more layers.
The most basic fuel cell is called a 3layer MEA (Membrane Electrode Assembly) and consists of an ion exchange membrane in the middle of two electrodes, typically made from sintered titanium or graphitized carbon plates. An anode and a cathode catalyst layer.
Hydrogen and Oxygen get in. Electricity (and water) gets out.
What fuels can be used in fuel cells?
Fuel cells offer flexibility in the fuel type that can be used. While hydrogen is the most common fuel source for fuel cells (hence the common name, hydrogen fuel cells), hydrogen-rich fuels such as natural gas and ammonia are also viable fuel sources.
Hydrogen: When produced using renewable electricity – like solar, wind and hydropower – hydrogen is completely decarbonized and produces zero emissions. Hydrogen fuel cells (i.e. fuel cells that are fueled by hydrogen) produce power, heat and water and release no carbon dioxide or other pollutants into the air.
Natural gas: As widespread production of green hydrogen is still in progress, natural gas is currently the most-used fuel to power fuel cells. In this case the fuel cells are not completely emission-free, but they do offer significantly lower emissions than other fuels, like oil and coal.
Ammonia: Ammonia is most used in agriculture as fertilizer. However, in recent years, several companies have been working to develop green ammonia. Green ammonia is made with hydrogen that comes from water electrolysis powered by alternative energy, making it another option for a low-carbon fuel.
Types of Fuel Cells and Their Applications: PEMFCs, SOFCs, DMFCs, AFCs, and PAFCs
Fuel cells are electrochemical devices that convert chemical energy into electrical energy. There are several different types of fuel cells, each with its own unique characteristics and applications. Here are a few things about some of the most common types of fuel cells:
Proton Exchange Membrane Fuel Cells (PEMFCs): These are the most common type of fuel cells and are widely used in automotive applications. They use a polymer electrolyte membrane and require hydrogen and oxygen as fuel and oxidant, respectively. PEMFCs are known for their high efficiency, low emissions, and fast start-up times.
Solid Oxide Fuel Cells (SOFCs): These fuel cells operate at high temperatures (typically above 800°C) and use a solid oxide electrolyte. They can operate on a variety of fuels, including hydrogen, natural gas, and biogas, making them suitable for a wide range of applications, including power generation and cogeneration.
Direct Methanol Fuel Cells (DMFCs): DMFCs are similar to PEMFCs but use methanol as a fuel instead of hydrogen. They are typically smaller and more compact than PEMFCs and are used in portable applications such as mobile phones and laptops.
Alkaline Fuel Cells (AFCs): These fuel cells use an alkaline electrolyte (usually potassium hydroxide) and were among the first types of fuel cells to be developed. They are primarily used in niche applications such as spacecraft and submarines.
Phosphoric Acid Fuel Cells (PAFCs): These fuel cells use phosphoric acid as an electrolyte and operate at temperatures of around 150°C. They are often used in stationary power generation applications, such as in hospitals and office buildings.
These are just a few examples of the many types of fuel cells that exist. Each type has its own unique characteristics and advantages, making them suitable for a range of different applications.