Electrochemical Materials
Electrochemical materials are materials that are capable of conducting electrical current through a redox reaction, i.e. an oxidation-reduction reaction. They are used in a wide range of applications where electrical conductivity and ion exchange properties are required.
Electrochemical materials include ion exchange membranes, carbon papers, carbon cloth, and conductive films, as well as other materials such as catalysts, electrodes, and separators. These materials play a crucial role in various electrochemical processes such as water treatment, fuel cells, batteries, and electroplating.
Frequently Asked Questions
What is the advantage of using electrochemical materials in fuel cells?
Electrochemical materials are essential for fuel cells, as they facilitate the transfer of ions and electrons between the electrodes and the electrolyte. They also play a role in catalyzing the electrochemical reaction, which is necessary for producing electrical energy from the fuel cell.
The use of electrochemical materials in fuel cells increases the efficiency and stability of the reaction, leading to improved performance and longer lifetimes for fuel cells..
What is the main use of conductive films in electrochemical processes?
Conductive films are used as separators in electrochemical processes such as batteries and fuel cells.
They provide electrical insulation between the electrodes while allowing ionic transport. This helps to maintain the stability and uniformity of the electrochemical reaction.
Learn More
Electrochemical Materials: The Key to Optimizing Electrochemical Processes
Electrochemical devices utilize chemical reactions to generate or consume electrical energy. They operate based on the principles of electrochemistry, which involves the movement of electrons and ions during chemical reactions. Electrochemical materials play a critical role in these devices due to their ability to efficiently convert and store energy, detect specific compounds, and perform other useful functions. As such, electrochemical materials find a wide range of applications across various industries, including water treatment, fuel cells, batteries, and electroplating.
Key Applications of Electrochemical Devices
Power generation using fuel cells
Fuel cells are electrochemical devices that convert chemical energy from a fuel directly into electrical energy without combustion.
Electrochemical sensing
Electrochemical sensors are used in environmental monitoring, medical diagnostics, food safety, and industrial applications. They can detect specific analytes such as gases, ions, and biomolecules, making them desirable for qualitative and quantitative analysis.
Environmental remediation
Electrochemical devices are also used for heavy metal removal and waste water treatment (electrocoagulation and electrooxidation systems).
Energy storage
Electrolyzers, batteries and supercapacitors are essential electrochemical devices used for energy storage. They are used in portable electronic devices, electric vehicles, renewable energy systems, including as solar and wind power storage, and grid-scale energy storage to manage the fluctuations in energy supply and demand.
Electrochemical Devices for Power Generation and Energy Storage Applications: Distinguishing between Fuel Cells and Electrolyzers
Fuel cells are electrochemical devices that convert chemical energy from a fuel directly into electrical energy without undergoing combustion. In fuel cells, a fuel is oxidized at the anode, releasing electrons, and an oxidant is reduced at the cathode, accepting electrons. The electrochemical reactions occur at separate electrodes. The generated electrical energy can be used to power various applications.
On the other hand, electrolyzers are electrochemical devices that use electrical energy to drive a non-spontaneous chemical reaction. Specifically, water electrolysis involves passing an electric potential to split water into its constituent gases, H2 and O2. Electrolyzers play a significant role in the development of the hydrogen economy, as they enable the large-scale production of hydrogen for energy storage and transportation purposes.
When fuel cells and electrolyzers are coupled in a close loop, a hybrid system (Hybrid Electrolyzer–Fuel cell system) is realized. This system is designed for a Power-to-Power application, where electrical energy is converted into hydrogen gas through water electrolysis and then converted back into electricity using a fuel cell when needed. This closed-loop configuration allows for energy storage and retrieval, making it a promising solution for grid stabilization and energy balancing.
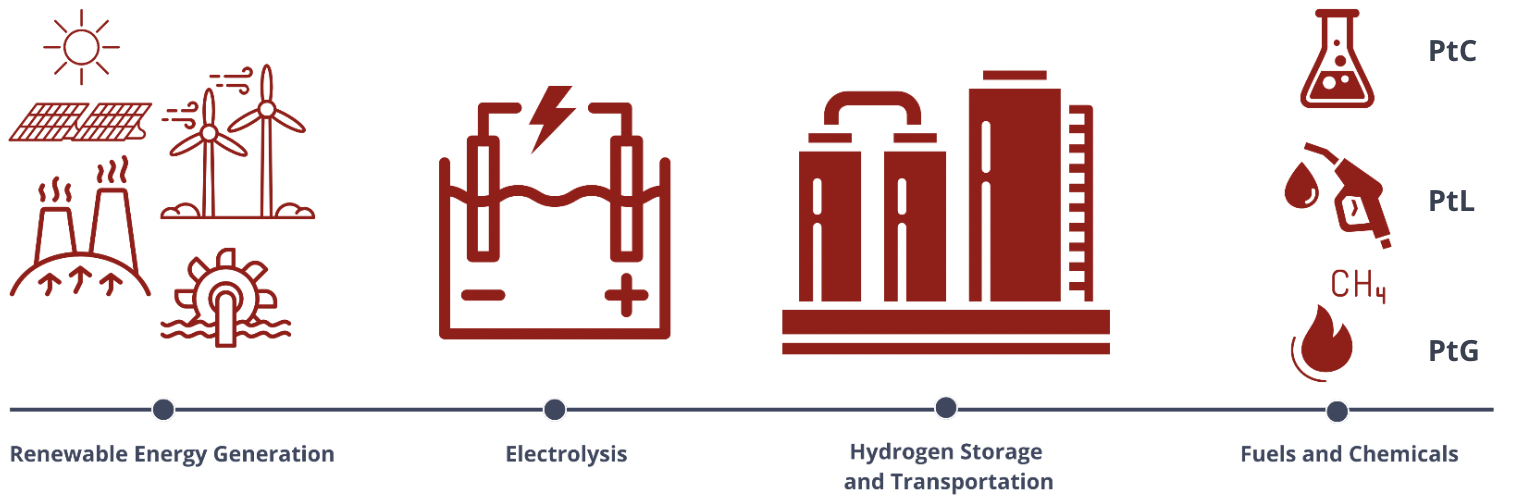
Aside from this, electrolyzer technology can also be employed for a Power-to-X application. In Power-to-X applications, electrical power, usually generated from renewable energy sources, is converted into different forms of energy carriers or valuable products. The "X" in Power-to-X represents the variety of products that can be obtained from the process, such as Power-to-Liquids (PtL), Power-to-Gas (PtG), and Power-to-Chemicals (PtC), among others. Each of these pathways serves as a means to store energy, distribute it efficiently, and utilize it in different applications.
Materials for Electrochemical Devices
At the forefront of electrochemical materials are ion exchange membranes, carbon papers and cloth, conductive films, and catalysts. These materials are designed to meet the demands of various electrochemical applications, and their properties and characteristics can be optimized to improve the efficiency and stability of the processes they are used in.
Ion Exchange Membranes
Ion exchange membranes are essential components in many electrochemical processes, as they are responsible for separating ions of different charges and regulating the flow of ions between two electrode compartments. These membranes are typically made of a polymer material and contain ion exchange sites that allow for the exchange of ions, ensuring the stability and efficiency of the electrochemical reaction.
Carbon Papers and Carbon Cloth
Carbon papers and carbon cloth are commonly used as electrodes in fuel cells and batteries. They provide a conductive substrate that facilitates the transfer of ions and electrons between the electrode and the electrolyte, as well as catalyze the electrochemical reaction. These materials are chosen for their high electrical conductivity, mechanical stability, and high surface area, which allows for a high catalytic activity.
Conductive Films
Conductive films are essential components in batteries and fuel cells, where they are used as separators. These films provide electrical insulation between the electrodes while allowing ionic transport, which helps to maintain the stability and uniformity of the electrochemical reaction. Conductive films can be made from a variety of materials, including polymers and metal oxides, and are chosen for their electrical conductivity, mechanical stability, and thermal stability.
Catalysts
Catalysts play a crucial role in the electrochemical reaction in fuel cells and batteries, as they increase the rate of the reaction. Catalysts are typically made from noble metals, such as platinum and palladium, and are chosen for their high catalytic activity and durability. The optimization of catalysts is critical to the performance and efficiency of fuel cells and batteries.
Electrochemical materials are the key to optimizing electrochemical processes. Whether it's ion exchange membranes, carbon papers and cloth, conductive films, or catalysts, these materials play a critical role in ensuring the stability and efficiency of these processes. For those seeking to improve the performance of their electrochemical systems, it is essential to understand the properties and characteristics of these materials and how they can be optimized for specific applications.








