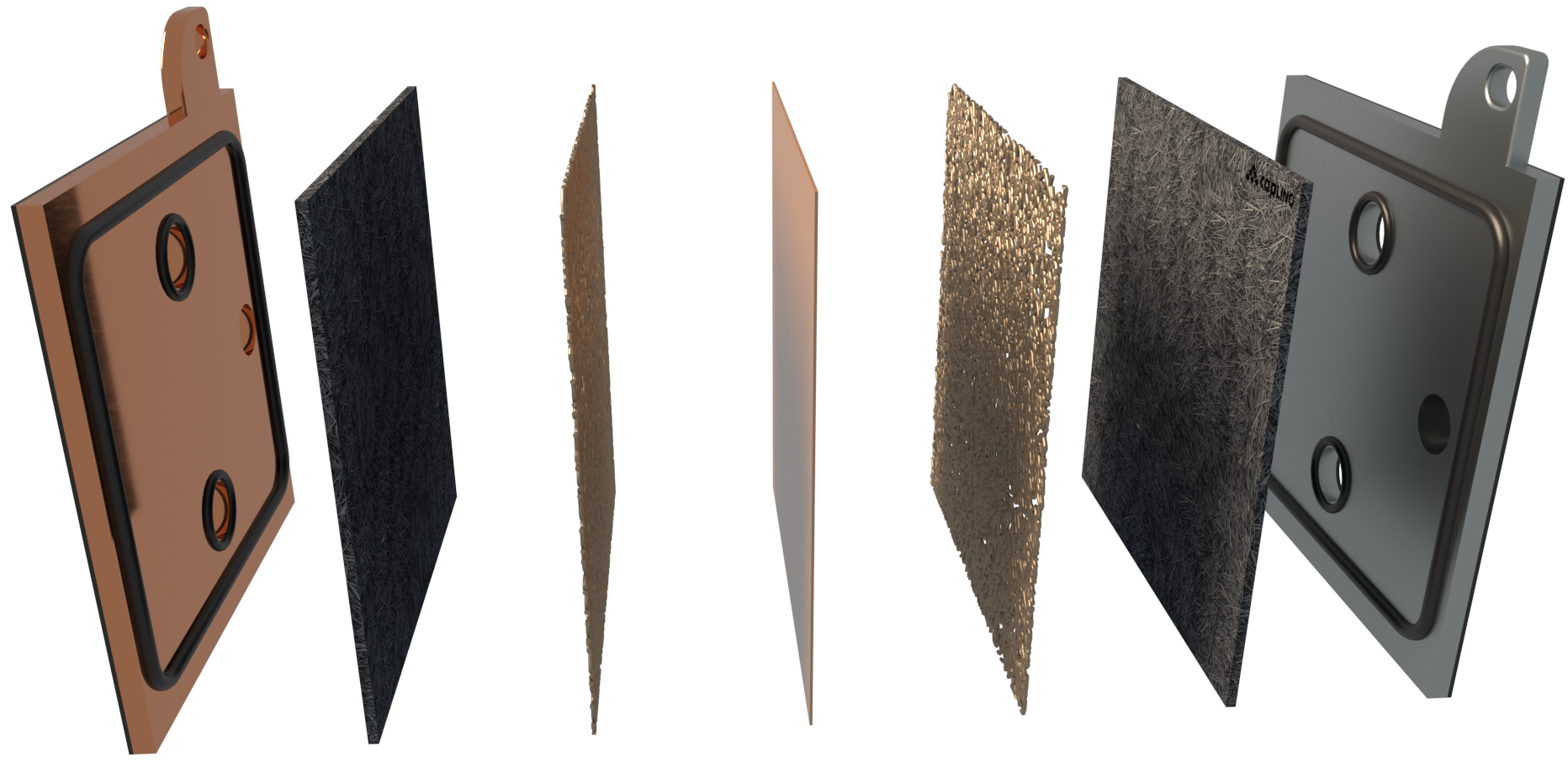AEMION+™ - AF3-CLF9-25
- CO2 Electrolysis
- 25 micron
- Non Woven PTFE
Product Description
AF3-CLF9-25 is an anion exchange membrane with a Non - woven PTFE reinforcement that has low ionic resistance, high electrical resistance, and strong chemical stability in solutions of both high and low pH, including concentrated alkaline solutions from 0.1M to 2 M at 90 °C.
AF3-CLF9-25 Anion Exchange Membranes (AEMs) with non-woven PTFE reinforcement are designed for use in various applications such as CO2 Electrolysis, fuel cells, and electrochemical processes. They allow the transport of negatively charged ions while blocking the passage of other ions.
AEMs reinforced with PTFE provide increased mechanical strength, improved chemical stability, enhanced thermal stability, increased flexibility, and high permselectivity. These AEMs are suitable for applications that require high-performance membranes that can withstand harsh conditions such as high temperatures, high pressure, and aggressive chemicals. They are typically used in electrochemical processes and water treatment applications.
Technical Specifications
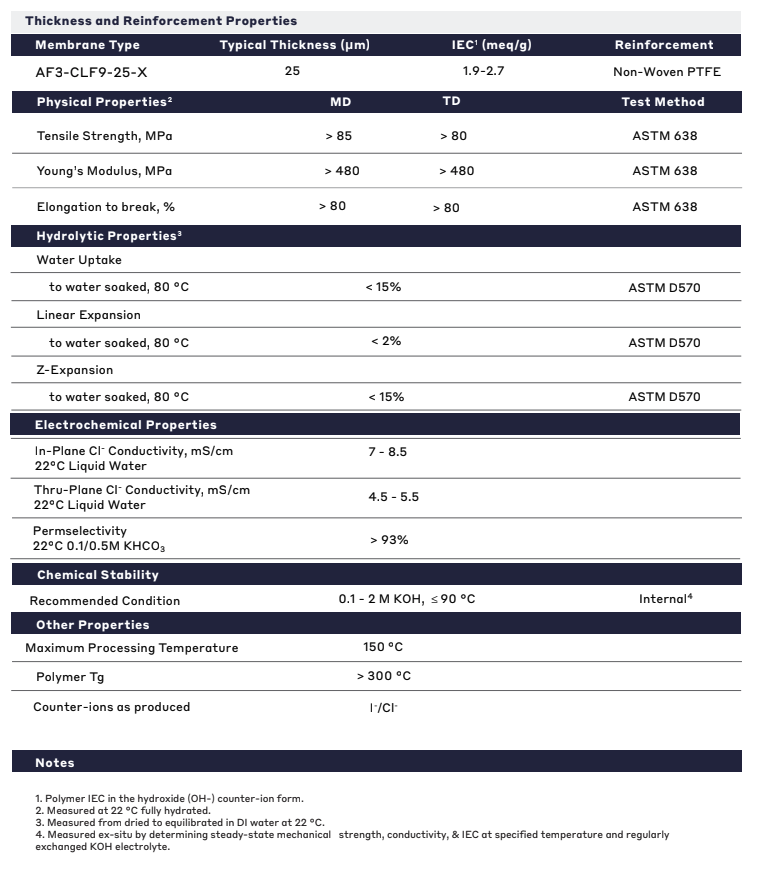
| General Properties | |
| Total Thickness Total Thickness Total thickness is taking into account all the films, coatings, adhesives, release liners and special layers and is the maximum thickness of a film or tape. | 25 μm |
| Thermal Properties | |
| Glass Transition Temperature (Tg) Glass Transition Temperature (Tg) The glass transition temperature for organic adhesives is a temperature region where the polymers change from glassy and brittle to soft and rubbery. Increasing the temperature further continues the softening process as the viscosity drops too. Temperatures between the glass transition temperature and below the decomposition point of the adhesive are the best region for bonding. The glass-transition temperature Tg of a material characterizes the range of temperatures over which this glass transition occurs. | 300 °C |
| Mechanical Properties | |
| Elongation Elongation Elongation is the process of lengthening something. It is a percentage that measures the initial, unstressed, length compared to the length of the material right before it breaks. It is commonly referred to as Ultimate Elongation or Tensile Elongation at break. | 80 % |
| Chemical Properties | |
| Ion Exchange Capacity (IEC) Ion Exchange Capacity (IEC) Ion-exchange capacity measures the ability of a material to undergo displacement of ions, previously attached into its structure, by oppositely charged ions. It is measured as the quantity of ions that can pass through a specific volume and a common unit is eq/L. In the case of an Ion-exchange polymer, it represents the total of active sites or functional groups responsible for the exchange and is the theoretical maximum amount of ions that we can load. | 1.9 - 2.7 meq/g |
| Physical Properties | |
| Young's modulus | 480 MPa |
| Hydrolytic Properties | |
| Linear Expansion | 2 % |
| Water Uptake | 15 % |
| Z-Expansion | 15 % |
| Electrochemical Properties | |
| In Plane Cl- Conductivity | 7 - 8.5 |
| Permselectivity Permselectivity Permselectivity is the term used to define the preferential permeation of certain ionic species through ion-exchange membranes. The permselectivity of a membrane is determined by the ratio of the flux of specific components to the total mass flux through the membrane under a given driving force. | 93 % |
| Thru Plane Cl- Conductivity | 4.5 - 5.5 |
Additional Information
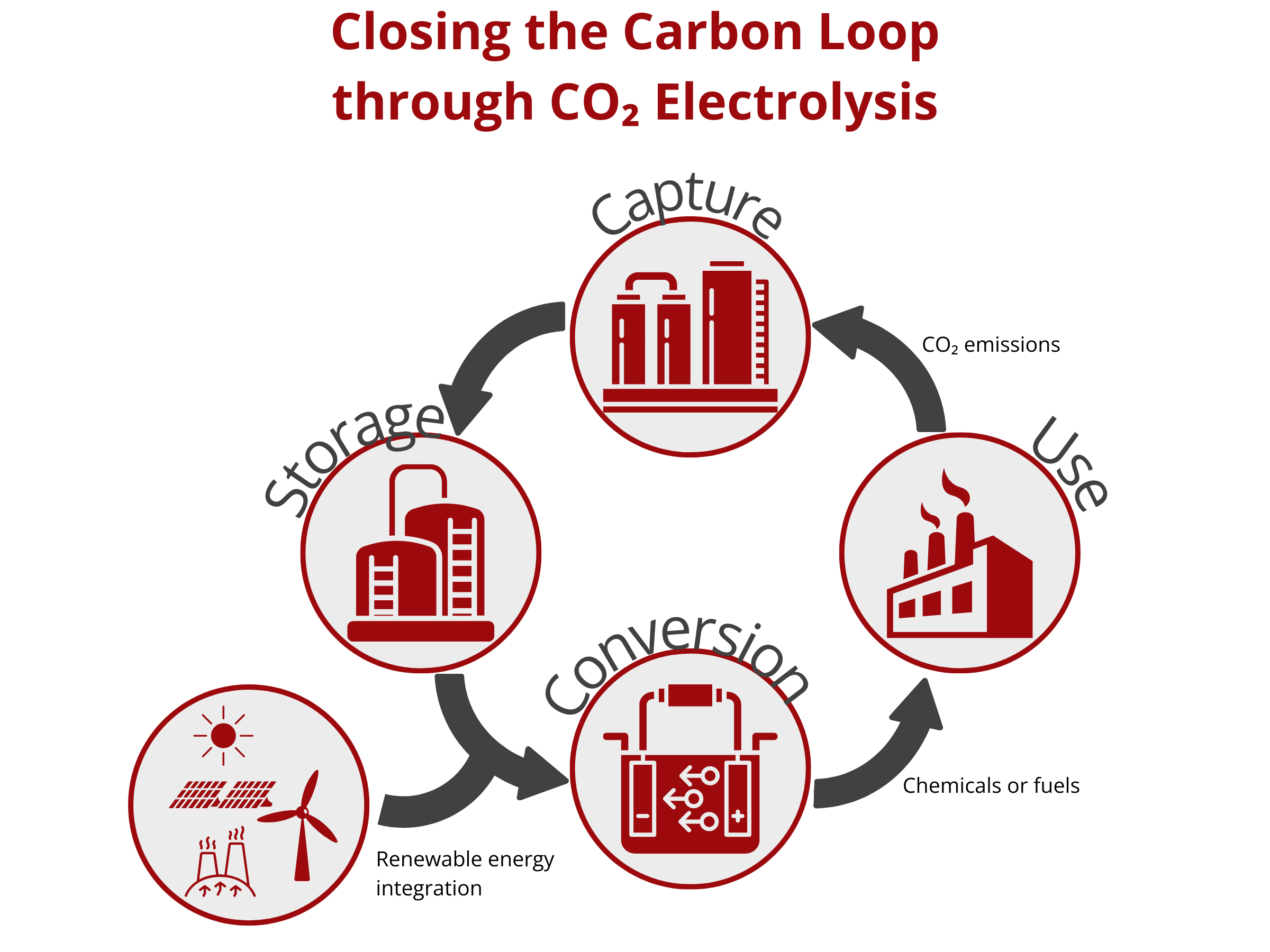
Closing the Carbon Loop through CO2 Electrolysis
CO2 emissions continue to increase as several industries continue to depend on fossil fuels. The atmospheric CO2 concentration has almost doubled after the Industrial Revolution (Current: >400 ppm, before the Industrial Revolution: 280 ppm). This increase translated to a global temperature rise of approximately 2 °C. To reduce the adverse effects of global warming to human life and property, the Intergovernmental Panel on Climate Change (IPCC) limits greenhouse gas emissions in such a way that the global temperature rise will decrease to 1.5 °C.
One promising way to decarbonize industries is to couple the heavy industrial processes with renewable energy. This route necessitates the use of efficient carbon capture and utilization strategies to sequester CO2 from the atmosphere or any other gaseous streams and then convert it to high-value chemicals and fuels. Among the different CO2 conversion technologies, CO2 electrolysis proves to be low cost, scalable, and sustainable. In contrast to the conventional thermochemical conversion method that requires huge energy input, the electrochemical conversion of CO2 using a CO2 electrolyzer can be operated under mild conditions and can be powered using renewable energy sources, such as solar, wind, tide, and geothermal energy. As such, electrochemical techniques can be carbon negative, thereby closing the carbon loop and possibly reducing CO2 atmospheric emission.
Non-woven PTFE (polytetrafluoroethylene) anion exchange membranes are a type of specialized material that is used in a range of applications, including water treatment and purification, as well as in the production of clean energy.
These membranes are made from a non-woven fabric of PTFE fibers, which are treated with a coating of anion exchange material. Anion exchange is a process in which negatively charged ions are exchanged for other negatively charged ions in a solution, allowing for the removal of impurities and contaminants.
The non-woven PTFE anion exchange membranes offer several advantages over traditional ion exchange resins, including high chemical resistance, high mechanical strength, and excellent thermal stability. They also have a high surface area-to-volume ratio, which allows for a high capacity for ion exchange.
These membranes are used in a range of applications, including water treatment and purification, where they can be used to remove contaminants such as heavy metals, nitrates, and other organic and inorganic impurities. They are also used in the production of clean energy, where they can be used in electrochemical systems such as fuel cells.
Overall, non-woven PTFE anion exchange membranes are a highly specialized material that offers excellent performance in a range of applications. Their high chemical resistance, mechanical strength, and thermal stability make them an ideal option for use in demanding environments where reliability and performance are critical.
What are the differences with woven PTFE membranes?
Anion Exchange Membranes (AEMs) with woven PTFE reinforcement are a type of ion exchange membrane that are used in various applications such as water treatment, fuel cells, and electrochemical processes. They allow the transport of negatively charged ions, such as chloride or hydroxide ions, across the membrane while blocking the passage of other ions. The AEMs are reinforced with a layer of PTFE (polytetrafluoroethylene) which is a hydrophobic material known for its chemical resistance, high temperature tolerance and mechanical strength.
The woven PTFE reinforcement provides several benefits to the AEMs such as:
- Increased mechanical strength: The PTFE reinforcement increases the AEM's tensile strength and durability, making it more resistant to mechanical damage.
- Improved chemical stability: The PTFE reinforcement provides a barrier against the penetration of aggressive chemicals, which can degrade the AEM.
- Enhanced thermal stability: PTFE can withstand high temperatures and has a low thermal expansion coefficient, which helps to maintain the AEM's stability in high-temperature applications.
- Increased flexibility: The woven PTFE reinforcement makes the AEM more flexible, which can be beneficial for certain applications where flexibility is important.
- High permselectivity: Woven PTFE reinforcement improves the permselectivity of AEMs, means, the ability of the membrane to selectively transport ions of a specific charge.
These AEMs are suitable for applications that require high-performance membranes that can withstand harsh conditions such as high temperatures, high pressure, and aggressive chemicals. They are typically used in electrochemical processes and water treatment applications.
Non Disclosure Agreement
This is a proprietary product from Ionomr, therefore, we require a signed NDA between the end customer and Ionomr before we are able to ship out the material, either for commercial use, or research.
This is done to prevent reverse engineering and public disclosure. The NDA does not restrict patenting anything, but does include the physical materials and their composition as confidential which prevents specific disclosure in a patent or otherwise but not calling out use of an AEM including Aemion by trade name. The test results are included as confidential information, and require approval from both parties to disclose. We are not concerned about blocking any publications, but would typically advise if we believe better results can be achieved.
We have a more explicit MTA (Materials Transfer Agreement) we use with academic/research organizations which outlines more detail around materials IP and publication.
Please find the NDA here and fill it in when contacting us for quotations.