AEMION+™-AF3-HWC9-70
- Water Electrolysis
- Acid Recovery and Salt Splitting
- 70 micron thick membrane with woven PEEK reinforcement
Product Description
AEMION+™-AF3-HWC9-70 is a breakthrough advanced polyimidazolium-based ion exchange membrane that enables the rapid growth of the hydrogen economy. Ionomr's Aemion+® anion exchange membranes are significantly more durable than our competitors', leading to thinner membranes, longer service life and reduced overall system costs.
AEMION+™ membranes have low ionic resistance and high electrical resistance. They are the first available commercial materials with excellent chemical stability in solutions of both high and low pH, and even in the harshest alkaline electrolysis systems (2–3M KOH, 80-100 °C). These advanced anion exchange membranes are frontiers in materials science with their unique hydrocarbon structure and good mechanical strength. All of these desirable properties unlock other potential applications that were previously constrained by the membrane’s poor durability and integrity. AEMION+™ is the enabling, platform material for the scale-up of AEMWE technology to industrial level.
AEMION+™-AF3-HWC9-70 is the thinner version of AEMION+™ - AF3-HWK9-75. The thickness of ion exchange membranes plays a crucial role in the performance and efficiency of water electrolyzers and fuel cells. Generally, thinner membranes are often preferred to maximize conductivity and efficiency. Therefore, thin membranes are more suitable in applications where high power density and rapid response are more critical. On the other hand, thicker membranes are preferred or applications requiring long-term stability and robustness. Optimizing the membrane thickness to your specific electrochemical process and operating conditions is the key to unlocking optimal performance.
With its excellent chemical stability at the full pH spectrum (0–14), AEMION+™-AF3-HWC9-70 can also be used in acid receovery and salt splitting applications. Aemion+® anion exchange membranes exhibit high oxidative tolerance, selectivity, and extended maintenance cycles, contributing to extended system lifetimes. AEMION+™-AF3-HWC9-70 demonstrates slightly lower proton blocking at high acid concentrations compared to other purpose-built AEMs. Still, it demonstrates significantly lower resistance and total power consumption per mole of acid, making them effective in concentrating applications (i.e., removal of total sulfate to 0 from a stream), and in use for acid concentration up to 5–7 wt%.
Technical Specifications
| General Properties | |
| Total Thickness Total Thickness Total thickness is taking into account all the films, coatings, adhesives, release liners and special layers and is the maximum thickness of a film or tape. | 70–75 μm |
| Thermal Properties | |
| Glass Transition Temperature (Tg) Glass Transition Temperature (Tg) The glass transition temperature for organic adhesives is a temperature region where the polymers change from glassy and brittle to soft and rubbery. Increasing the temperature further continues the softening process as the viscosity drops too. Temperatures between the glass transition temperature and below the decomposition point of the adhesive are the best region for bonding. The glass-transition temperature Tg of a material characterizes the range of temperatures over which this glass transition occurs. | >300 °C |
| Mechanical Properties | |
| Elongation Elongation Elongation is the process of lengthening something. It is a percentage that measures the initial, unstressed, length compared to the length of the material right before it breaks. It is commonly referred to as Ultimate Elongation or Tensile Elongation at break. | >15 % |
| Physical Properties | |
| Young's modulus | 600–850 MPa |
| Hydrolytic Properties | |
| Linear Expansion | 3 (22 °C) % |
| Water Uptake | 15 (22 °C) % |
| Z-Expansion | 5 (22 °C) % |
| Electrochemical Properties | |
| Thru Plane Cl- Conductivity | >5 |
Additional Information
AEMION+® Enables Large-Scale Commercial Development of Water Electrolyzers
Alkaline water electrolysis (AWE) uses a liquid KOH/NaOH electrolyte between electrodes separated by a porous diaphragm. It benefits from low-cost catalysts but suffers from higher internal resistance and typically lower current densities.
Proton exchange membrane water electrolysis (PEMWE) is compact and efficient due to high H+ conductivity, but the acidic environment drives reliance on PGM catalysts—raising CAPEX and OPEX.
This motivates a technology that blends AWE’s inexpensive catalysts with PEMWE’s compact zero-gap design: alkaline anion exchange membrane water electrolysis (AEMWE/AAEMWE). Using an anion exchange membrane enables low-cost catalysts while maintaining a compact architecture. At current scales, AWE and PEMWE CAPEX exceed DOE targets; AEMWE offers a pathway to lower CAPEX and competitive green hydrogen. Developing high-performance, stable AEMs is central to this transition.
| Description | AWE | PEMWE | AEMWE |
|---|---|---|---|
| Technology readiness | ✓ | ✓ | ✕ |
| Non-PGM loading | ✓ | ✕ | ✓ |
| Long-term stability | ✓ | – | ✕ |
| MW scale | ✓ | ✓ | ✕ |
| Compact design | ✕ | ✓ | ✓ |
| Current density | ✕ | ✓ | ✓ |
| Cost effective | – | ✕ | ✓ |
| Operating pressure | ✕ | ✓ | ✓ |
| Non-corrosive environment | ✕ | ✕ | ✓ |
At 1 MW, AWE ($1279/kW) and PEMWE ($1168/kW) are similar, while AEMWE with AEMION+® is lower at $931/kW and $926/kW (without/with PGM). At 5 MW, all costs drop but AEMWE remains lowest ($444–459/kW). The pathway to low-cost, sustainable electrolysis is enabled by continued AEMWE advances. | 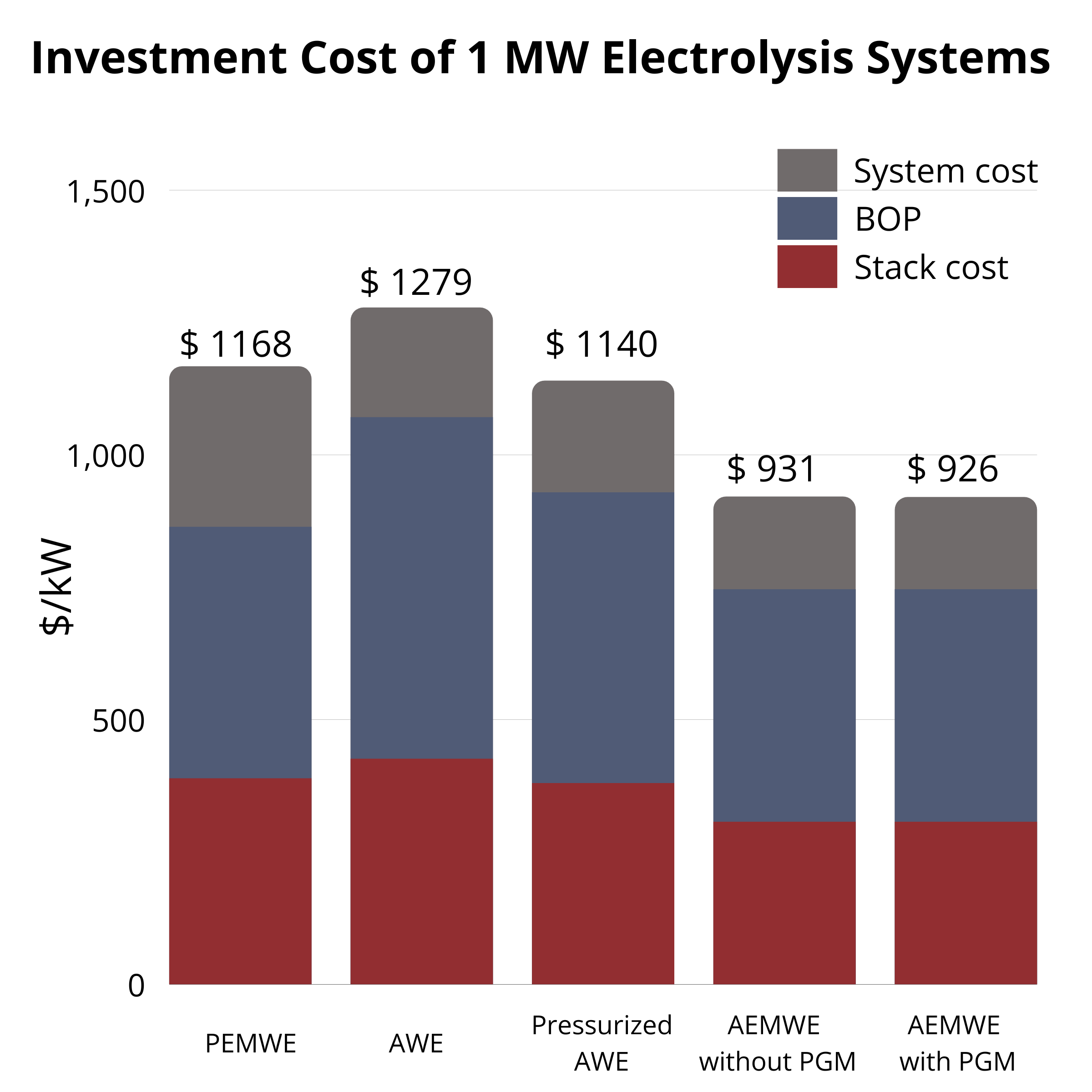 CAPEX at 5 MW | 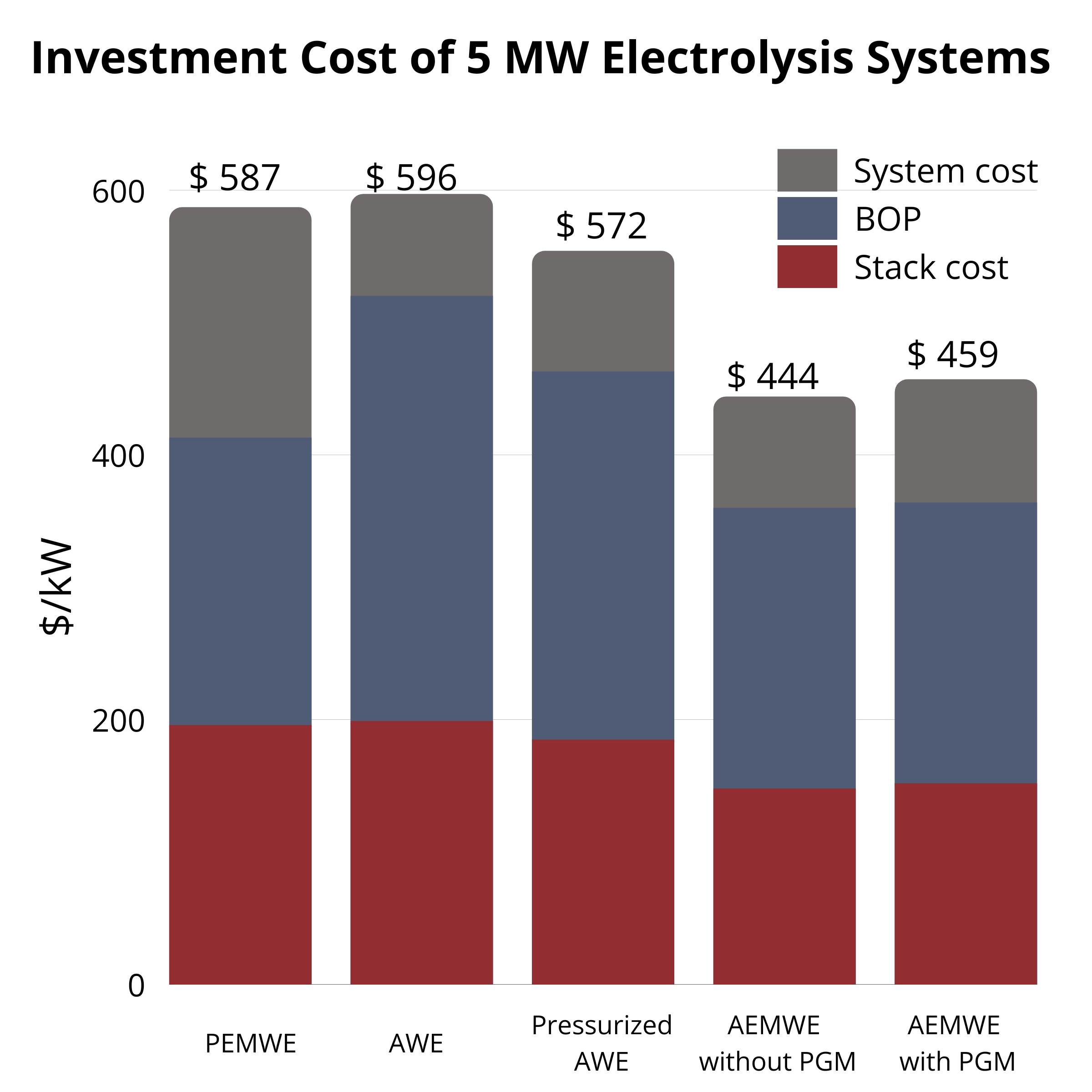 CAPEX at 1 MW |
Zero Degradation Found in Latest Test of Aemion+® Alkaline Membrane
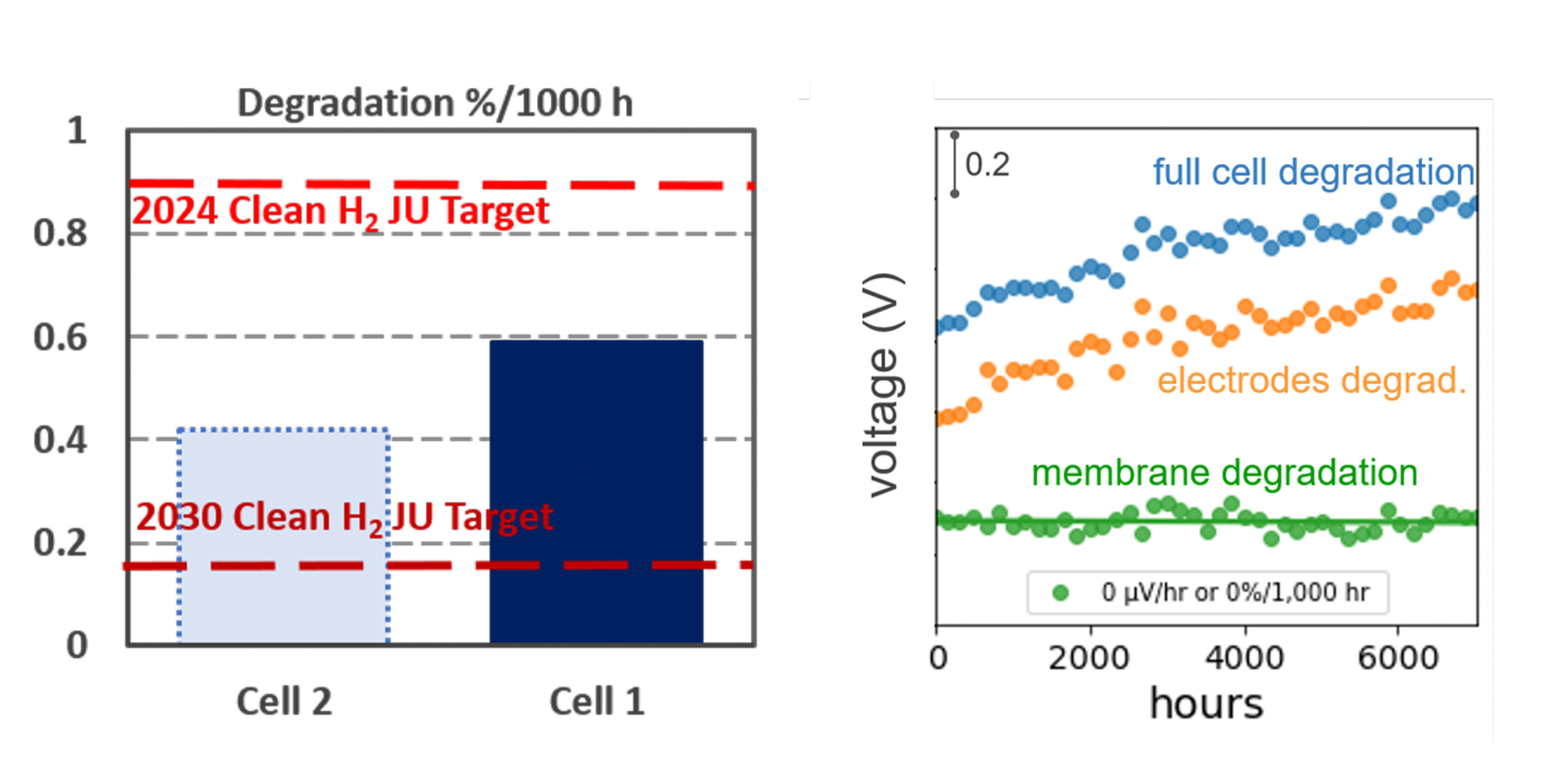
Background. A 50 cm2 cell using Aemion+® membranes and long-life alkaline electrodes has been on-test for 2500 h at the 2024 EU target current density (0.6 A cm−2), building on >7500 h prior tests with <0.6%/1000 h post break-in system degradation and <0.05%/1000 h measurable membrane degradation.
Results. Cells showed no detectable membrane degradation (<0.05%), with total system degradation <0.4%/1000 h—surpassing the EU Clean Hydrogen JU 2024 target of 0.9%/1000 h. Most losses came from electrodes/porous metal transport elements; removing the porous metal improved outcomes. Ionomr continues optimization with NREL under Shell GCxN. NREL reports Ionomr MEAs >1 A cm−2 at 2 V in hydroxide electrolyte without iridium and stable >500 h. See the latest press release.
Key Specifications of AF3-HWC9-70-X
| Membrane Type | Typical Thickness (µm) | IEC1 (meq/g) | Reinforcement |
|---|---|---|---|
| AF3-HWC9-70-X | 70 – 75 nominal | 2.1 – 2.6 | Woven PEEK |
| Property | MD / Value | TD | Test Method |
|---|---|---|---|
| Physical Properties2 | |||
| Tensile Strength (MPa) | 40–70 | 40–70 | ASTM 638 |
| Young’s Modulus (MPa) | 600–850 | 600–850 | ASTM 638 |
| Elongation to Break (%) | > 15 | > 15 | ASTM 638 |
| Hydrolytic Properties | |||
| Water Uptake (%) — 22 °C | < 15 | ASTM D570 | |
| Water Uptake (%) — 80 °C | < 15 | ||
| Linear Expansion (%) — 22 °C | < 3 | ASTM D570 | |
| Linear Expansion (%) — 80 °C | < 3 | ||
| Z-Expansion (%) — 22 °C | < 5 | ASTM D570 | |
| Z-Expansion (%) — 80 °C | < 7 | ||
| Electrochemical Properties | |||
| Through-plane Cl− Conductivity (mS·cm−1) | > 5 | Internal3 | |
| Hydrogen Permeability (N µL·cm−2·min−1·bar−1) | < 3.2 | Internal4 | |
| Chemical Stability | |||
| Recommended Operating Condition | 1 M KOH, 70 °C | Internal5 | |
| Other Properties | |||
| Maximum Processing Temperature (°C) | 150 | Internal6 | |
| Polymer Tg (°C) | > 300 | ASTM E1131 / ISO 11358 | |
| Counter-ions as Produced | I− / Cl− | — | |
- Polymer IEC in the hydroxide (OH−) counter-ion form.
- Measured at 22 °C, fully hydrated.
- Conditioned in 1 M NaCl for 24 h, then DI H2O for 6 h; tested fully submerged at room temperature.
- Hydrogen permeability measured electrochemically under internal reference conditions; verify in end-use systems.
- Guidance from steady-state measurements of strength, conductivity, and IEC in exchanged KOH electrolyte.
- Tg determined by TGA at 2 °C/min in the as-produced form; polymer can be rendered stable > 200 °C—contact Ionomr for higher-temperature use.
AEMION+® Reinforced Membranes Pre-treatment Guide
Membranes ship dry in chloride/iodide form. Depending on application, assembly may be done in the dry form or after pre-treatment. Exchange to the operating ionic form while assembled, as required by your device chemistry.
The following protocol outlines the recommended activation and handling steps for water electrolysis applications. Adjust electrolyte volume, temperature, and duration as necessary for your system scale and QA criteria.
Recommended Activation & Handling Steps before use in Water Electrolysis
Activation 1 — Iodide/Chloride → Nitrate Form Exchange
Depending on the assembly method, perform the iodide/chloride-to-nitrate exchange either ex situ (wet) or in situ (dry) using one of the protocols below.
| ACTIVATION STEP 1 — For wet membrane assembly: Iodide/Chloride → Nitrate form ex situ | |||||
|---|---|---|---|---|---|
| Protocol | Temp (°C) | Electrolyte | Volume ratio (mLelectrolyte/cm²AEM) | Recirculation? | Min time (hours) |
| Standard (room temp.) | RT | 3 M NaNO₃ | 0.5 | No | ≥ 2 |
| ACTIVATION STEP 1 — For dry membrane assembly: Iodide/Chloride → Nitrate form in situ | |||||
|---|---|---|---|---|---|
| Protocol | Temp (°C) | Electrolyte | Volume ratio (mLelectrolyte/cm²AEM) | Recirculation? | Min time (hours) |
| Standard (room temp.) | RT | 1 M NaNO₃ | 30 | Yes, 100 mL/min | ≥ 6 |
Activation 2: Nitrate → Hydroxide
Convert the nitrate form to hydroxide under the following standard conditions:
| ACTIVATION STEP 2 — Nitrate to Hydroxide Form | |||||
|---|---|---|---|---|---|
| Protocol | Temp (°C) | Electrolyte | Volume ratio (mLelectrolyte/cm²AEM) | Stirring? | Min time (hours) |
| Standard (room temp.) | RT | 1 M KOH | 2 | No | ≥ 4 |
Check conversion & electrical readiness
Verify ionic form by XRF/EDS or similar analytical method. Beginning-of-life HFR should be ≤ 200 mΩ·cm².
Mounting
Prefer mounting wet to avoid differential stresses from drying.
Recommended Activation & Handling Steps before use in Fuel Cells
For AEM fuel cell applications use the following general pre-treatment approach:
- Soak for at least 24 hours, and up to 36–48 hours is recommended.
- Exchange into KOH first may aid subsequent exchanges (e.g., sulfate).
Spray-Coating Catalyst Layers onto AEMION+ Membranes (CCM Preparation)
Optimized process for spray-coating catalyst layers directly onto ion-exchange membranes (e.g., Aemion⁺) using a Sono-Tek ExactaCoat or similar system
Quick overview (click to expand)
- Preheat to ~70 °C, fix membrane flat, avoid air gaps.
- Ink flow ≤ 0.8 mL min⁻¹; head speed ≤ 75 mm min⁻¹.
- Multiple light passes; rotate 90° periodically; repeat for second side.
- Dry gently; avoid excessive heat or strong airflow.
Preparation
Preheat to ~70 °C. Fix membrane flat with small (heat-resistant) tape at corners.
- Use a porous heat-transfer layer to avoid air gaps.
- 0.5 cm uncoated border; ≥ 2 cm mask overlap.
- Mild tension or a template frame to prevent curl.
Ink Loading
Load ≈ 25 mL ink, purge air, and use toggle to verify steady flow and a consistent spray pattern.
Coating and Feed / Flow Rate
- Flow: ≤ 0.8 mL min−1
- Head speed: ≤ 75 mm min−1
- Keep surface at ~70 °C throughout.
- Prefer multiple light passes to avoid pooling.
Layer Uniformity
- Pause every 1–2 runs for visual inspection.
- Adjust flow/pattern if unevenness appears.
- Rotate the membrane 90° every 5–10 runs.
- Repeat to target loading.
Coating the Second Side
After reaching target loading, flip, re-mask, align with the first side, and repeat with the same parameters.
Post-Coating Drying
Dry gently until touch-dry. With high-boiling solvents, extend time slightly; avoid excessive heat or strong airflow that can deform the CCM.
Notes & Cautions
- Do not exceed 0.8 mL min−1 flow or 75 mm min−1 head speed.
- Use templates/support frames during ion-exchange and coating to minimize curling.
- Pooling or streaks mean over-deposition → reduce flow or passes.
- Membranes must be clean and lightly hydrated but free of surface moisture before coating.
Removal of the Membrane from the Backing Layer
- With clean gloved hands, hold the membrane on its backing layer.
- Using a thumb or finger, rub against the corner edge of the membrane to produce separation from the backing layer.
- Once corner separation is achieved, carefully and gently pull the membrane from the backing while holding the backer on a clean, dry surface.
- Support the membrane as you peel until it has been fully removed.
- With clean gloves, wet a portion of the membrane/backing edge with de-ionized water to aid separation. Repeat the primary method.
- If separation does not start, spritz a small amount of water near the edge and try again.
For coating AEMION® membranes after removing from the backing layer
Ensure that the membrane remains flat.
A powder-coating masking tape can be used to overlap the membrane edges prior to coating to stabilize the membrane and minimize stress lines.
Use caution when removing tape after coating, as tears may occur. Cutting off the taped section is an alternative.
Use caution to remove tape from the membrane after the coating process.
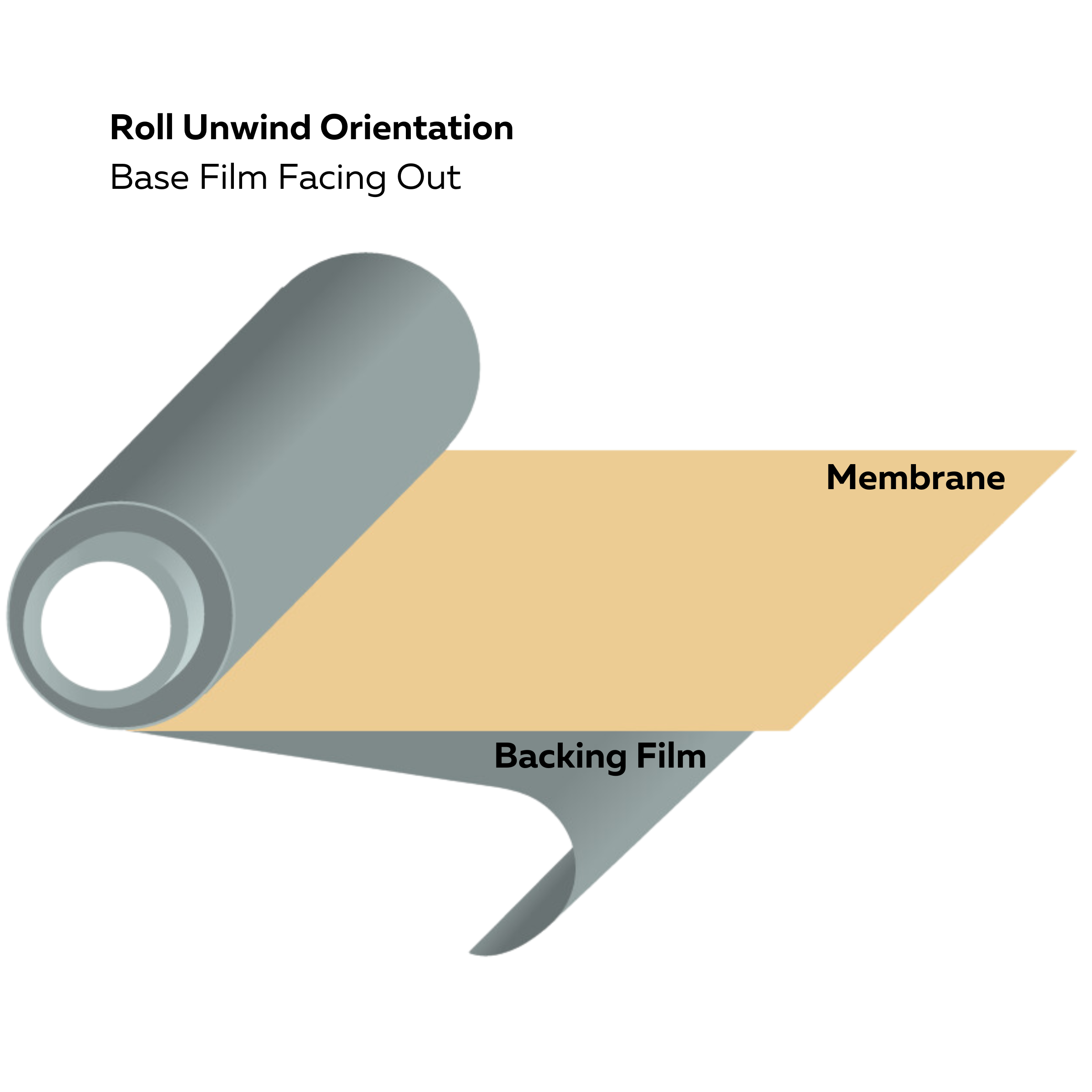
Roll unwind orientation (base film facing out)
Related Presentations
Related Blogs

How Pretreatment Affects Ion Exchange Membranes for Electrochemical Devices
This blog explores how pretreatment processes influence the performance of ion exchange membranes in electrochemical devices, highlighting the importance of proper conditioning to achieve consistent efficiency and durability.
Non-Disclosure Agreement
This is a proprietary product from Ionomr; a signed NDA between the end customer and Ionomr is required before shipment for either commercial use or research.
The NDA prevents reverse engineering and public disclosure. It does not restrict patenting, but treats the physical materials and their composition as confidential—preventing specific disclosure in a patent or otherwise—while still allowing reference to an AEM (e.g., Aemion) by trade name. Test results are confidential and require approval from both parties for disclosure. We are not concerned about blocking publications and will advise if better results appear achievable.
We also use a more explicit Materials Transfer Agreement (MTA) for academic or research organizations that further details materials IP and publication. Please find the NDA here and include it when requesting quotations.