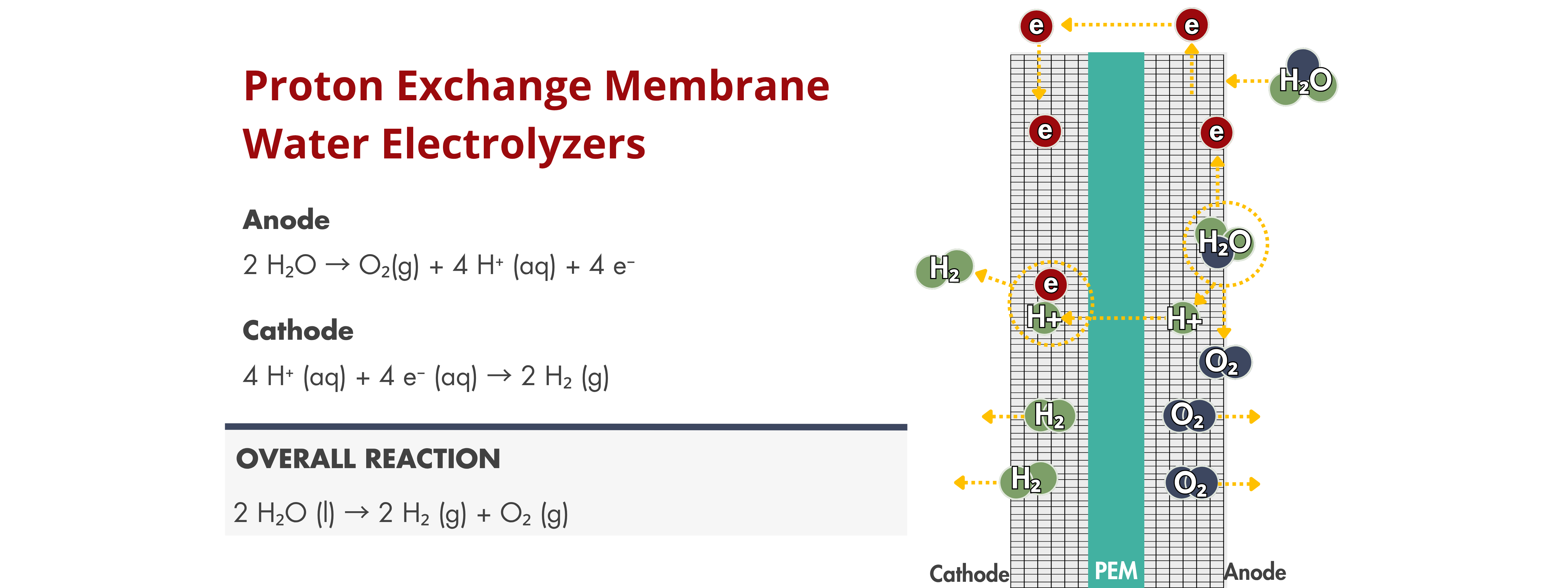
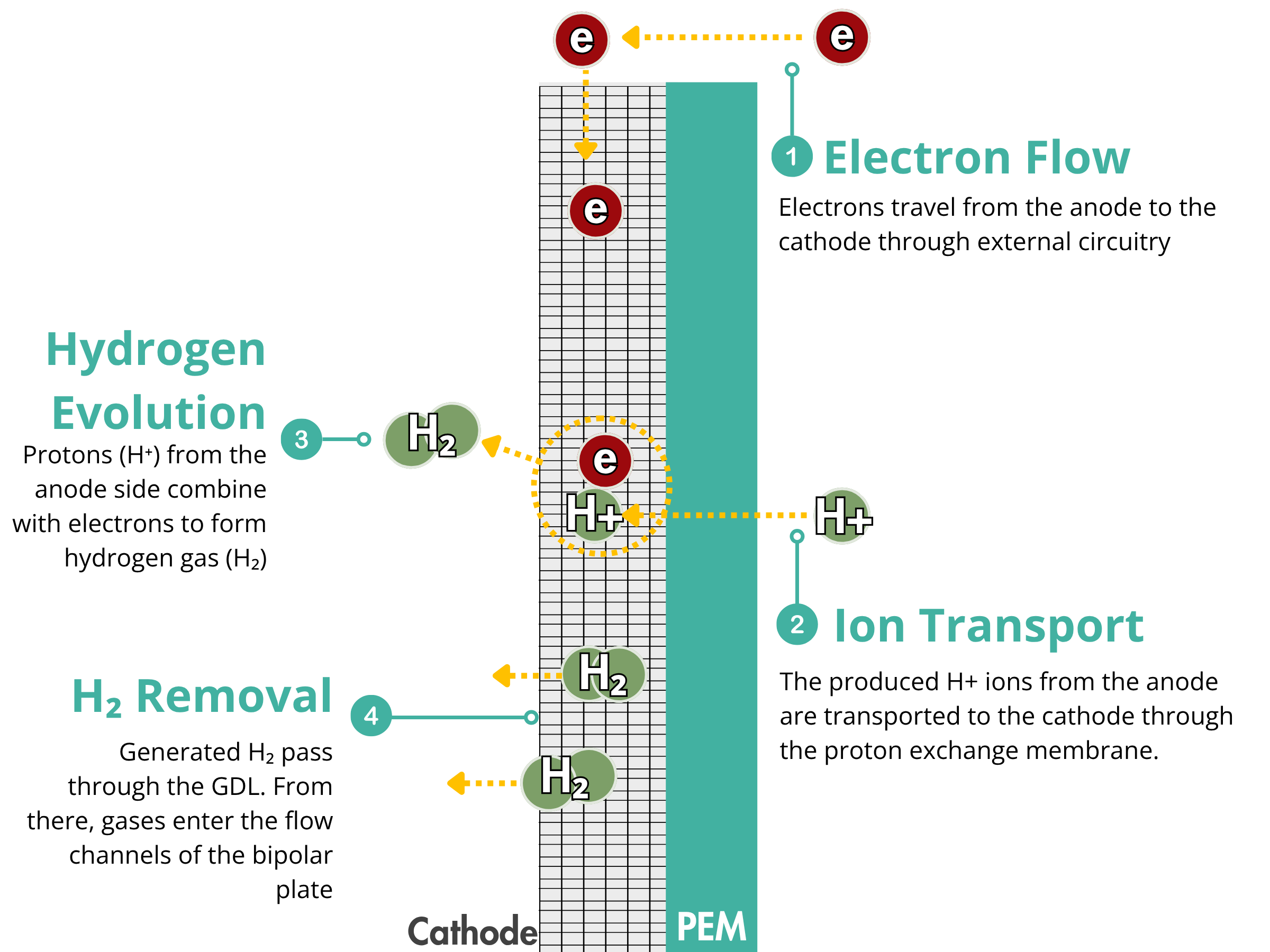
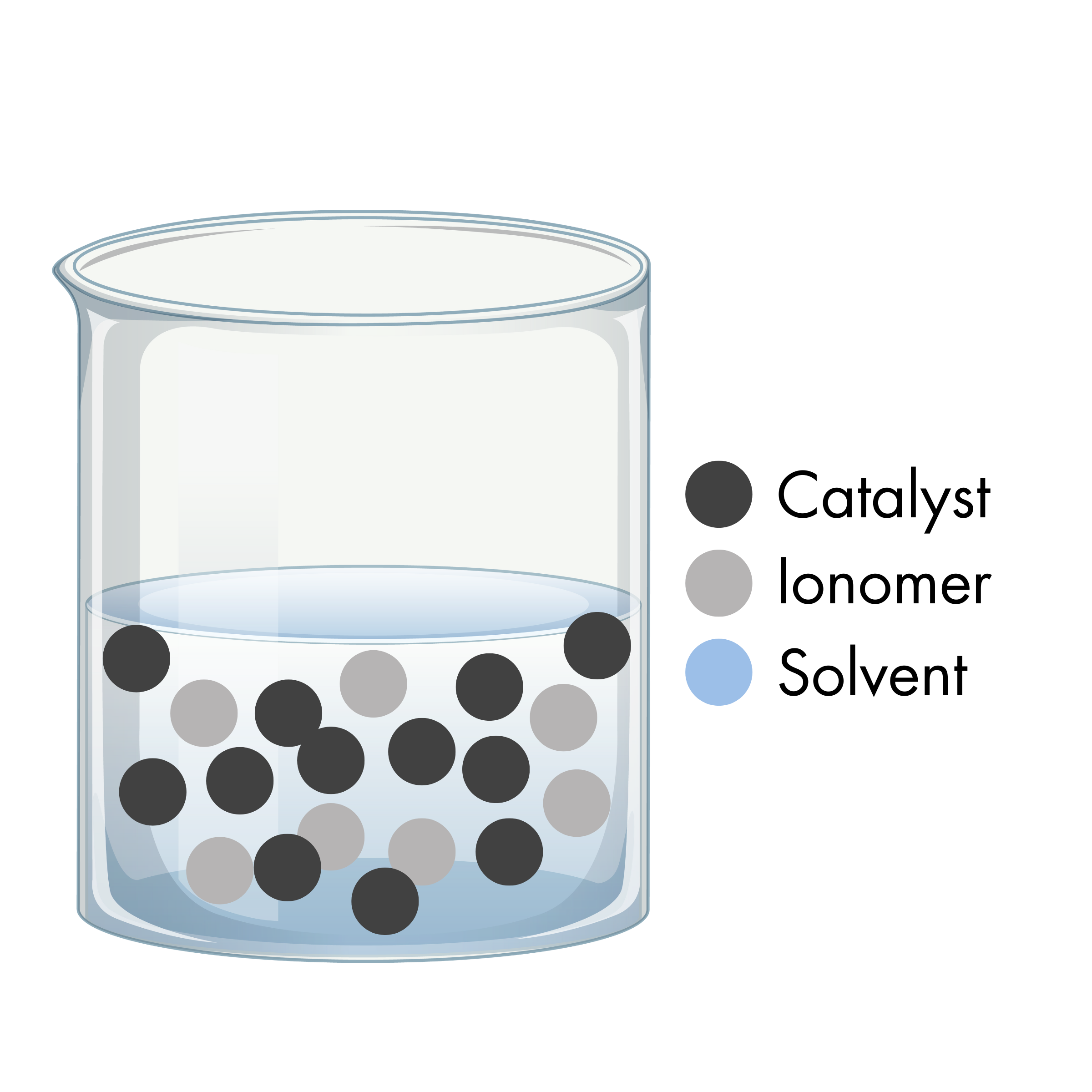
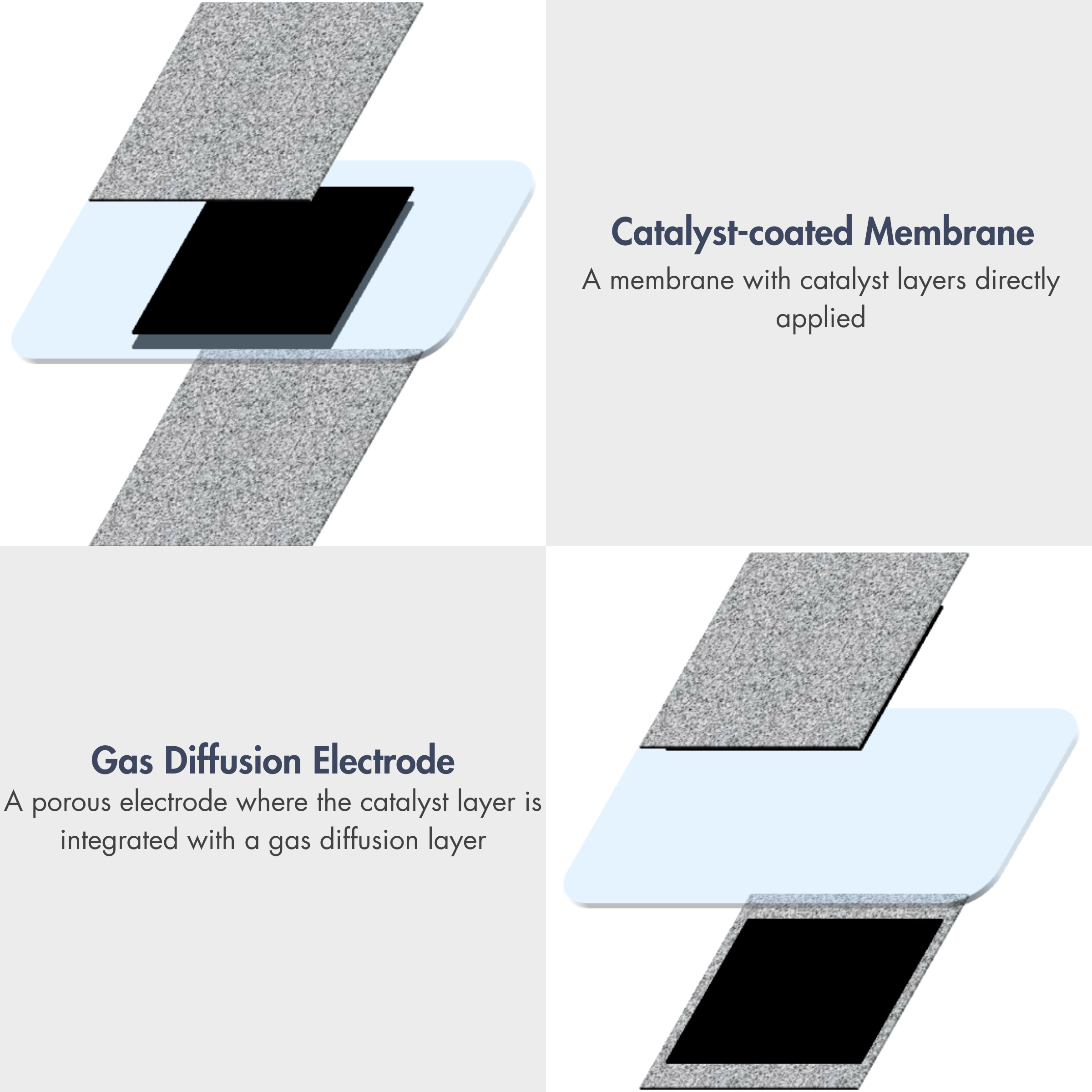
PEM Electrolyzers
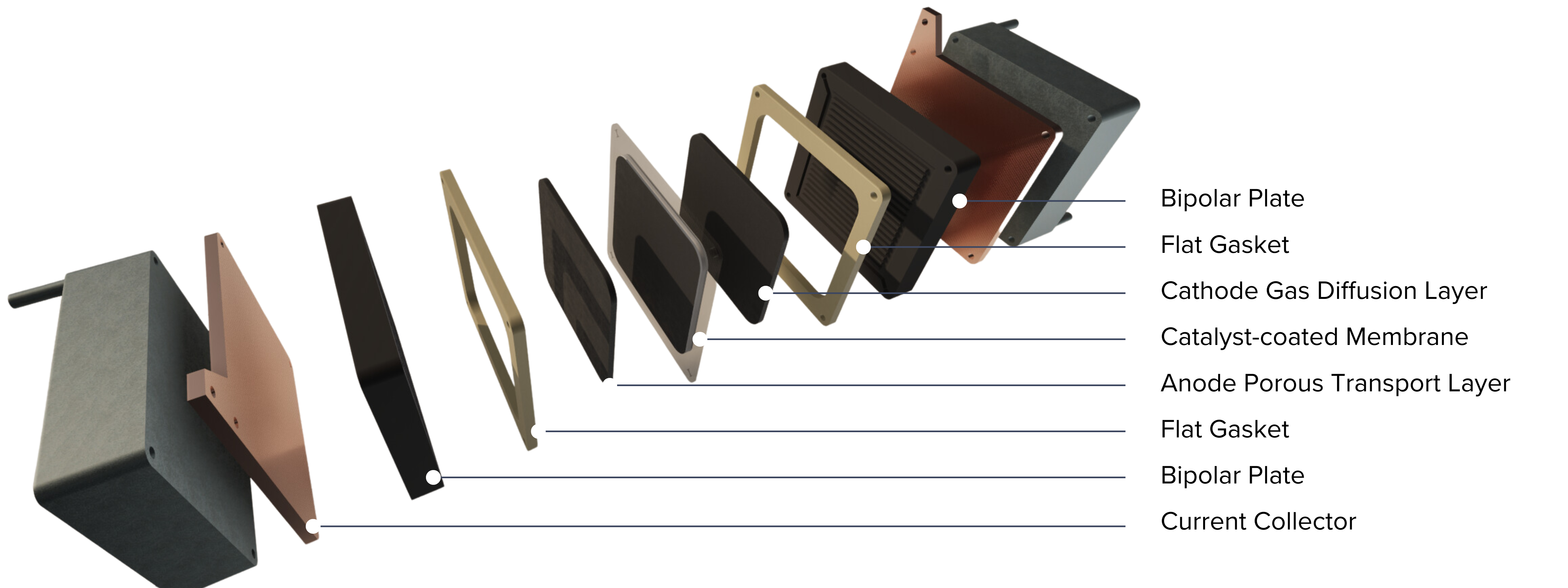
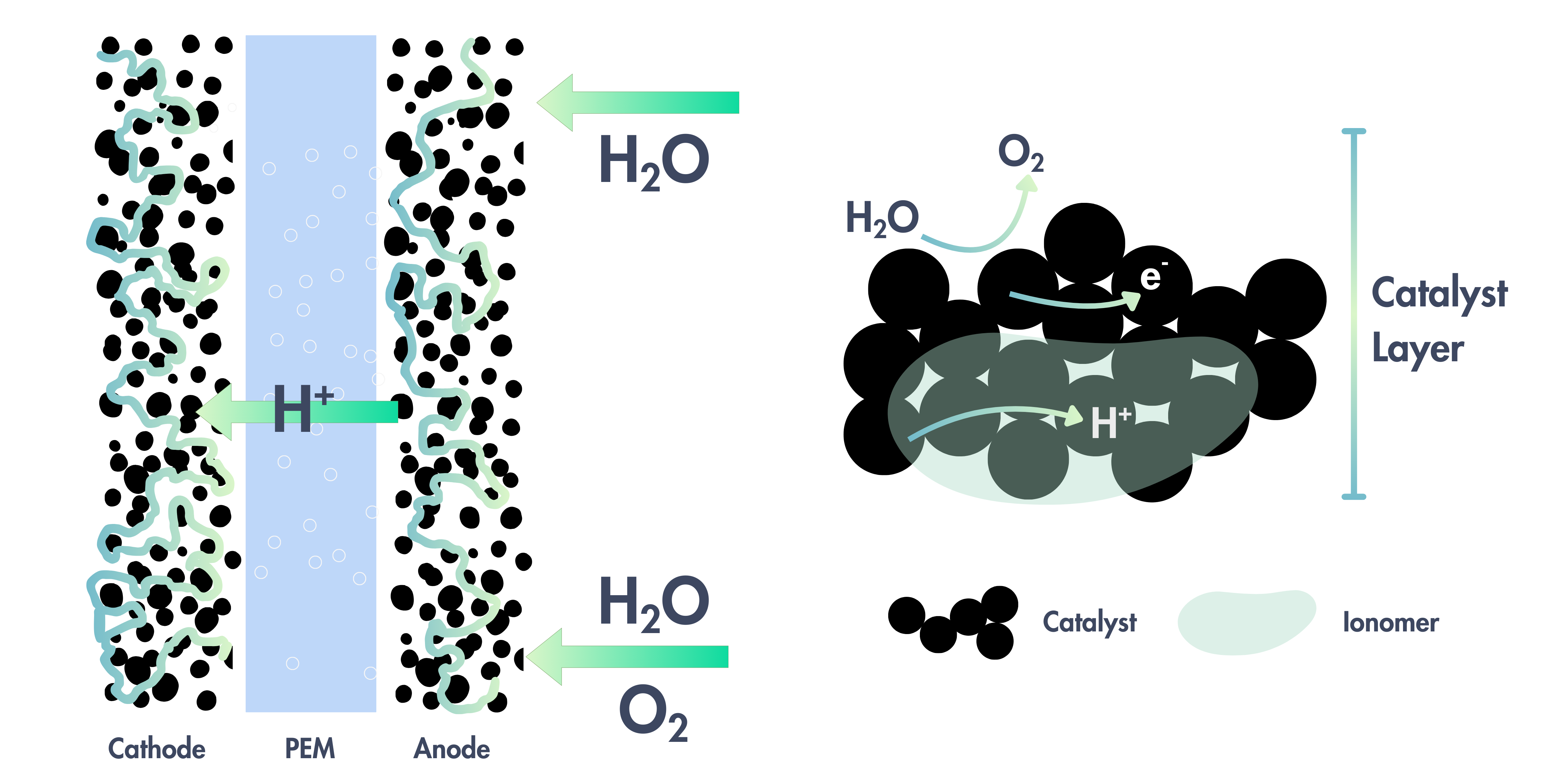
Cathode Gas Diffusion Layers and Anode Porous Transport Layers
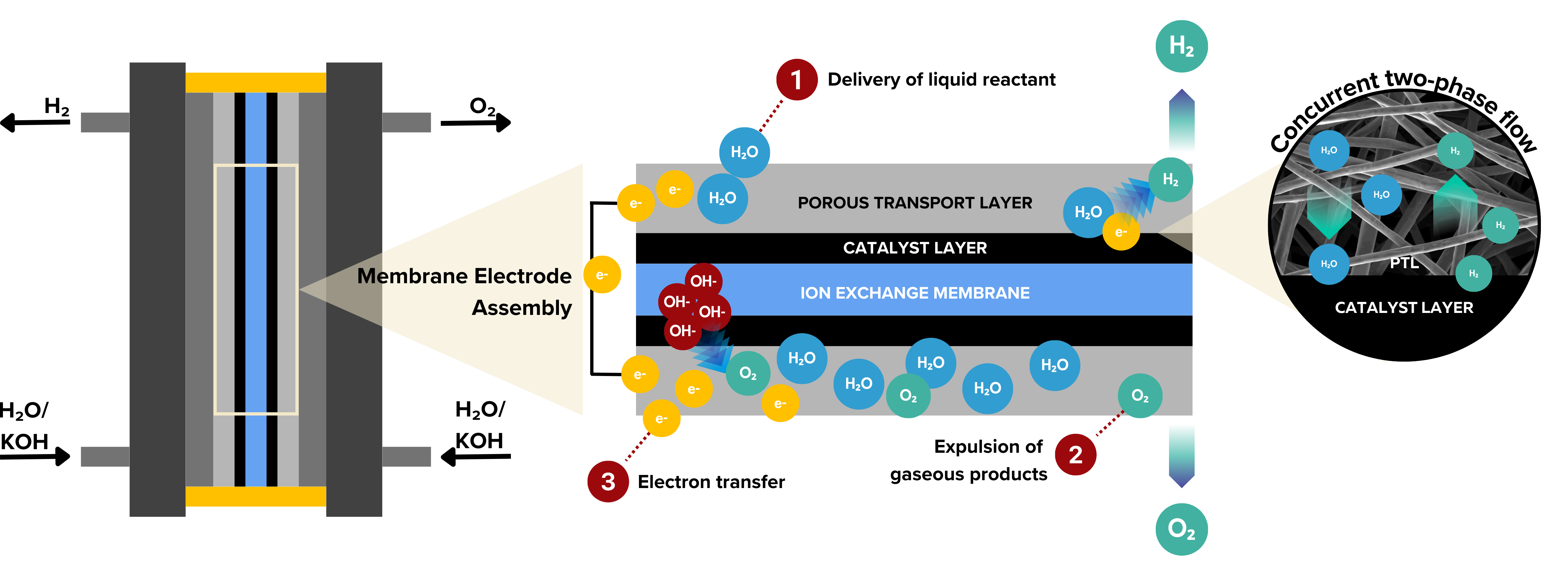
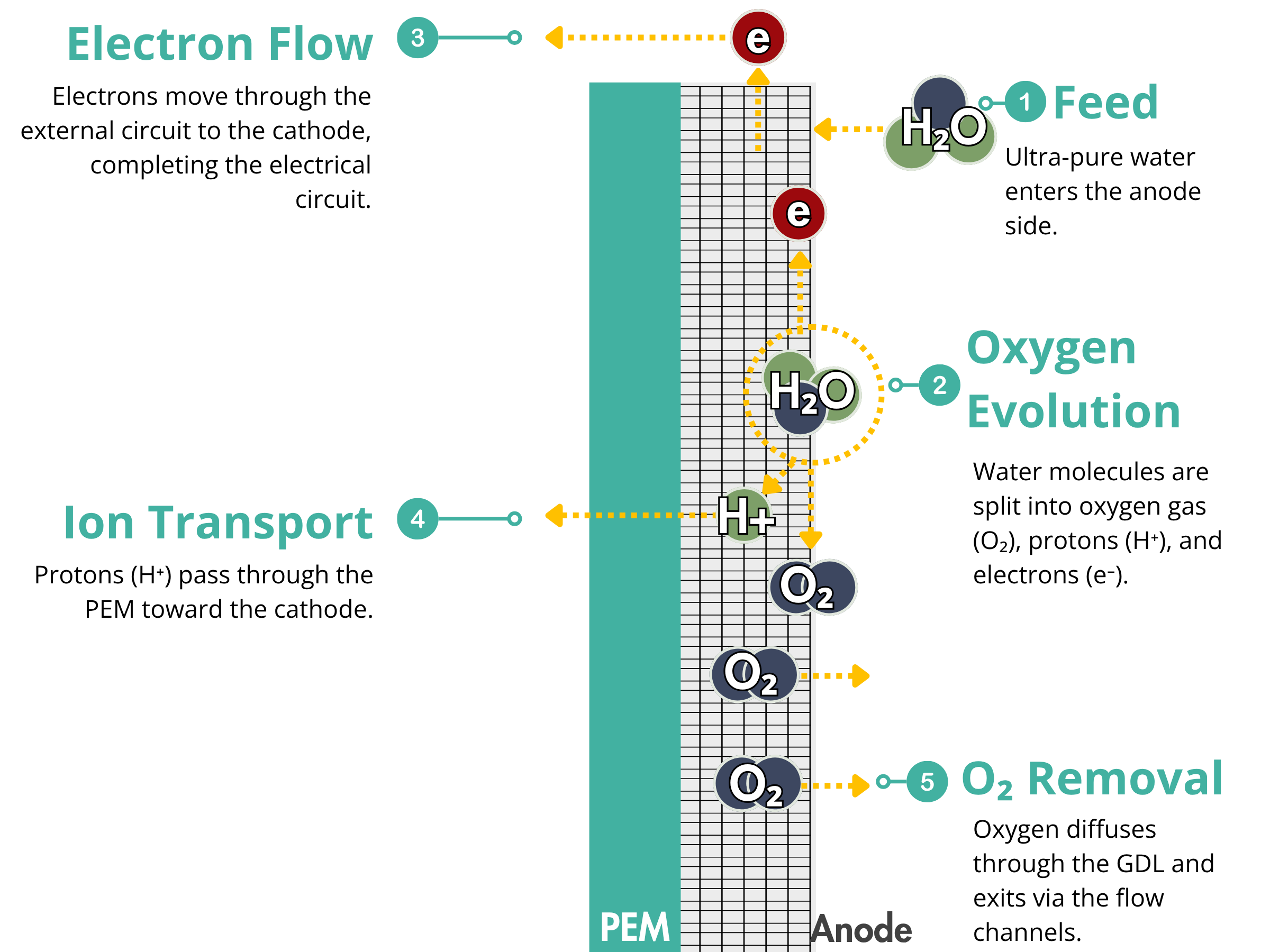
Porous transport layers (PTLs) and gas diffusion layers (GDLs) are important components in water electrolyzer stacks. They play several key roles:
Reactant feed and product removal
They help water reach the catalyst and allow hydrogen and oxygen to escape. Poor gas removal limits water supply and can reduce performance.
Electron transport
PTLs and GDLs carry electrons between the catalyst layer and the bipolar plate for efficient hydrogen production.
Heat management
They help spread and release heat to prevent hot spots and membrane damage.
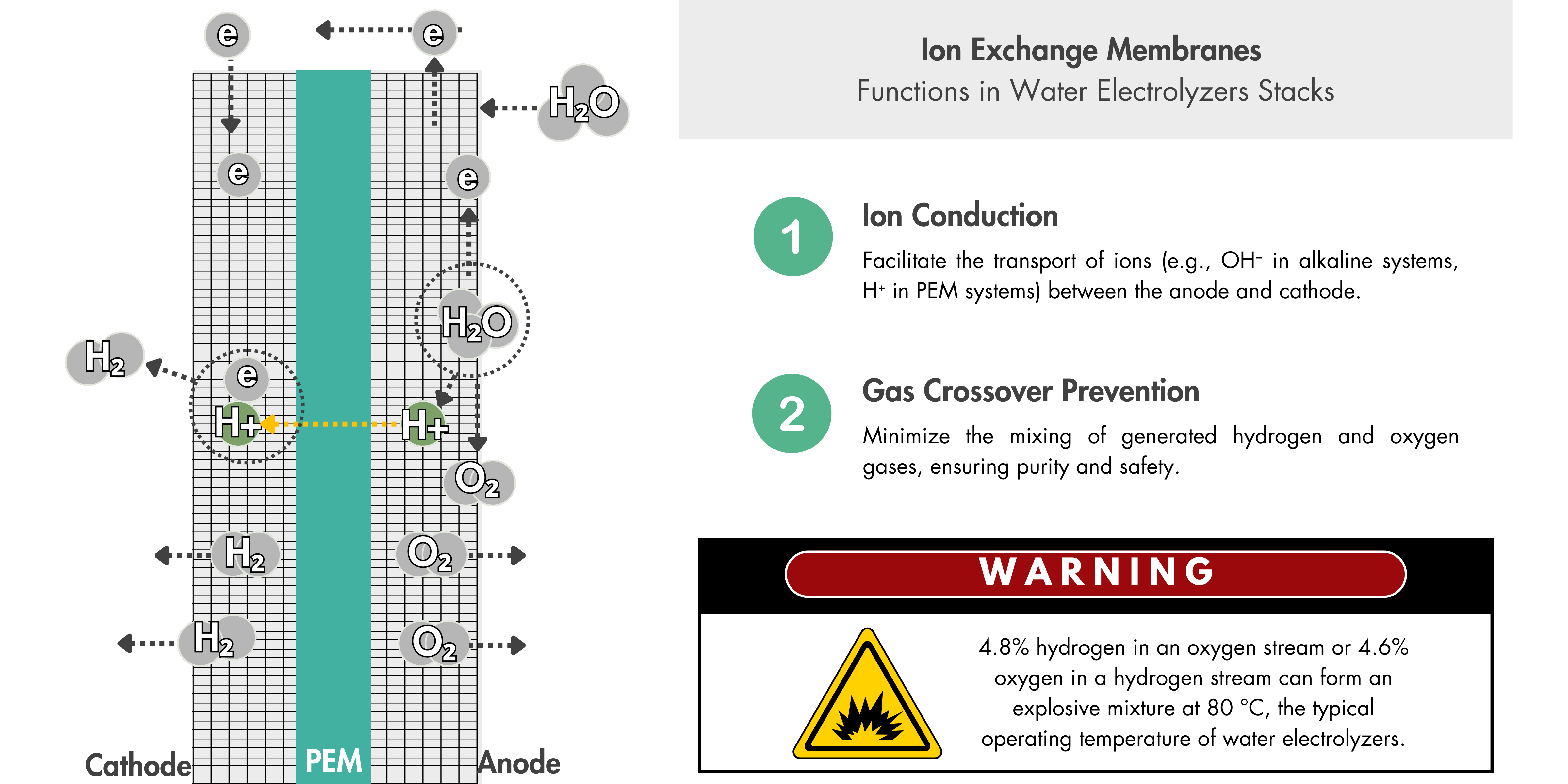
The choice of material to be used at the anode and cathode depends on the operating environment and requirements at each electrode.
| Side | Layer | Typical Material | Operating Conditions | Reason |
|---|---|---|---|---|
| Cathode | Gas Diffusion Layer (GDL) | Carbon | ~0 V vs. RHE, reducing environment, acidic pH (0–1) | Stable under reducing conditions, good conductivity, and cost-effective |
| Anode | Porous Transport Layer (PTL) | Titanium | ~1.6–2.0 V vs. RHE, oxidizing environment, acidic pH (0–1) | Excellent corrosion resistance under acidic and oxidative conditions |
Find a more in-depth explanation of material choices and their performance in water electrolyzer stacks in
LINQCELL Graphitized Carbon Fiber Plates are made by layering carbon fibers or particles with a conductive binder. CAPLINQ's carbon fibers are made from polyacrylonitrile fibers, which are then graphitized by heating the material to high temperatures in an inert atmosphere to convert it into graphite. These are thicker versions of carbon fiber papers.
Featured Products
1.5 mm thickness
- 1.5mm - 1500 um - 59mil
- Alternative to Spectracarb 6060
- Also available in a more compressible version (GDL1500B)
1.85 mm thickness
- 1.85mm - 1850 um - 73mil
- Alternative to Spectracarb 7590
2.9 mm thickness
- Thickest graphitized carbon fiber plate
- Basis weight can be as high as 1800 g/m2
- Low voltage loss (27 mV)
Key Features and Benefits of LINQCELLTM Graphitized Carbon Fiber Panels
- Low resistance, which minimizes energy losses and improves overall fuel cell efficiency
- Customizable GDL solutions to meet specific performance needs
- High corrosion resistance, making them durable in acidic conditions of fuel cells
- High mechanical strength, critical for maintaining integrity under high-pressure conditions
- Customizable compressibility
From Spectracarb to LINQCELL: Decades of GDL Expertise
CAPLINQ has been involved with graphitized carbon papers since the early 2000s—first as a partner of Spectracarb, then as a key technical resource for customers in the fuel cell and electrolyzer markets. Our deep understanding of Spectracarb’s products and processes allowed us to smoothly transition and build the LINQCELL line when Spectracarb was discontinued. With years of hands-on experience and a deep understanding of GDL materials, we’ve supported customers through industry changes and continue to provide reliable, application-driven solutions.
History of CAPLINQ, Spectracarb and LINQCELL →
Watch our presentation on PEM electrolyzers as current collectors, in Hannover Messe 2016.
Titanium is the preferred material for the anode porous transport layers (PTLs) of PEM water electrolyzers due to its excellent corrosion resistance in the highly oxidative and acidic environment on the anode side. It forms a stable oxide layer that protects against degradation while maintaining mechanical strength and stability under pressure and temperature.
LINQCELL™ Sintered Titanium Fiber Felts are produced from titanium wires drawn to a micron-scale diameter. These wires are stacked using a specialized laying method, then subjected to pressure and placed in a high-temperature vacuum environment for sintering. This fuses the nodes between fibers, forming a three-dimensional network structure.
- Grades: Ta0 (≥99.9% purity), Ta1 (Grade 1), Ta2 (Grade 2)
- Porosity: 50–80%
- Thickness: 0.20 mm – 1.5 mm
- Fiber diameter: 20–25 μm
- Max dimensions: 120 cm × 120 cm
- Optional platinum coating: Both sides, up to 0.50 μm
Also available: LINQCELL Sintered Titanium Powder
Frequently Asked Questions about PEM Water Electrolyzers
PEM electrolyzers are used for producing high-purity hydrogen in applications such as green hydrogen generation, hydrogen fueling stations, power-to-gas systems, synthetic fuel production, and industrial gas supply. Their dynamic response and compact design make them ideal for renewable energy integration.
What water quality is required for PEM electrolyzers?PEM electrolyzers require deionized water with a resistivity ≥1 MΩ·cm. Poor water quality can lead to membrane degradation, catalyst poisoning, and loss of efficiency, so high-purity water is essential for reliable operation.
PEM electrolyzers use acidic membranes and require noble metal catalysts like iridium and platinum. AEM electrolyzers operate in alkaline conditions and can use non-precious metals. PEM systems are more mature, with higher performance, while AEM offers potential for lower cost but is still emerging.
Can PEM electrolyzers operate intermittently?Yes. PEM electrolyzers have fast ramp-up times and excellent dynamic response, allowing them to efficiently operate under fluctuating power input from renewable energy sources like solar or wind.
Featured Presentation: CAPLINQ Product Offerings
CAPLINQ Materials for PEM Water Electrolyzers
Curious about how the right materials can improve electrolyzer performance? This quick presentation walks you through CAPLINQ’s lineup for PEM water electrolyzers: what they’re made of, how they perform, and where they fit best. Whether you’re optimizing for efficiency, durability, or both, these materials are engineered to keep up.
Got questions or need help choosing the right materials for your PEM water electrolyzer stacks? Reach out to us!
Contact Us →Presentations
Types of Low-Temperature Electrolyzers
This presentation introduces the main types of low-temperature electrolyzers—systems that operate below 150 °C and are widely used for green hydrogen production. It covers the core technologies, including alkaline, PEM, and AEM electrolyzers, and highlights their operating principles, key materials, benefits, and challenges.
Related Blogs

What are the Differences Between Alkaline, PEM, and AEM Water Electrolyzers?
This blog explores the key differences between alkaline, PEM (proton exchange membrane), and AEM (anion exchange membrane) water electrolyzers. It breaks down how each technology works, compares their materials and operating conditions, and highlights their respective advantages and limitations.

What Are the Differences Between Sintered Metal Powders and Sintered Metal Fiber Felts?
This blog explores the differences between sintered metal powders and sintered metal fiber felts, focusing on their structure, permeability, and performance in various applications. It also provides insights into the structure of sintered nickel fibers, helping readers understand what makes them suitable for alkaline water electrolyzers.

How Will the Impending PFAS-ban Affect Proton Exchange Membranes for Water Electrolyzers and Fuel Cells?
This blog discusses the potential impact of the impending PFAS ban on proton exchange membranes (PEMs) used in water electrolyzers and fuel cells. It examines why PFAS-based materials have been widely used, what challenges the ban presents for current membrane technologies, and how the industry is responding with PFAS-free alternatives.